��Ŀ����
��0.100mol/L�IJ�����Һ���ζ�δ֪Ũ�ȵĸ��������Һ��������Ӧ�Ļ�ѧ����ʽΪ��2KMnO4+5H2C2O4+3H2SO4=K2SO4+2MnSO4+10CO2��+8H2O��
��1�����������ҺӦװ�� �����ʽ����ʽ�����ζ����У�
��2������ʵ������д������в�������ʹ����KMnO4Ũ��ƫ�͵��� ��
A��ʢװKMnO4��Һ�ĵζ�����ˮϴ��δ��KMnO4��Һ��ϴ
B��ʢ������Һ�ĵζ��ܵζ�ǰ���첿�������ݣ��ζ��յ�ʱ������
C���ζ�ʱ��������Һ����ƿ��
D����ƿ��ˮϴ֮��δ�ô���Һ��ϴ
��3��ÿ��ȷ��ȡ20.00mL�ữ��KMnO4��Һ�ζ�������Һ��Ϊ��ɫ�Ұ�����ڲ���ԭʱ������H2C2O4��Һ25.00mL����βⶨ��ƽ��ֵ������KMnO4��Һ��Ũ��Ϊ ��
��4�����������һ����Ҫ�������������������ᡢ������������ʣ���֪H2O2����H2SO4�ữ��KMnO4��Ӧ�����ӷ���ʽΪ��2MnO_-+5H2O2+6H+=2Mn2++8H2O+5O2������KMnO4��Һδ���ữ����MnO_-�Ļ�ԭ����ΪMnO2����д�������ӷ���ʽ ��
��1�����������ҺӦװ��
��2������ʵ������д������в�������ʹ����KMnO4Ũ��ƫ�͵���
A��ʢװKMnO4��Һ�ĵζ�����ˮϴ��δ��KMnO4��Һ��ϴ
B��ʢ������Һ�ĵζ��ܵζ�ǰ���첿�������ݣ��ζ��յ�ʱ������
C���ζ�ʱ��������Һ����ƿ��
D����ƿ��ˮϴ֮��δ�ô���Һ��ϴ
��3��ÿ��ȷ��ȡ20.00mL�ữ��KMnO4��Һ�ζ�������Һ��Ϊ��ɫ�Ұ�����ڲ���ԭʱ������H2C2O4��Һ25.00mL����βⶨ��ƽ��ֵ������KMnO4��Һ��Ũ��Ϊ
��4�����������һ����Ҫ�������������������ᡢ������������ʣ���֪H2O2����H2SO4�ữ��KMnO4��Ӧ�����ӷ���ʽΪ��2MnO_-+5H2O2+6H+=2Mn2++8H2O+5O2������KMnO4��Һδ���ữ����MnO_-�Ļ�ԭ����ΪMnO2����д�������ӷ���ʽ
��������1�����ݸ��������Һ����ǿ������ѡ��ζ������ͣ�
��2���ɹ�ϵʽ2KMnO4��5H2C2O4��֪V��KMnO4��?c��KMnO4��=
V��H2C2O4��?c��H2C2O4������c��KMnO4��=
��Ȼ��ݴ˷����жϣ�
��3����KMnO4��Һ��Ũ��Ϊc�����ݹ�ϵʽ2KMnO4��5H2C2O4���㣻
��4������ϡ�����ữ��MnO4-����ԭΪMnO2��˫��ˮ�������������������ݵ���غ��֪���������������ɣ��ٸ���ԭ���غ��ж��Ƿ���ˮ���ɣ���ƽ��д��
��2���ɹ�ϵʽ2KMnO4��5H2C2O4��֪V��KMnO4��?c��KMnO4��=
| 2 |
| 5 |
| ||
| V(KMnO4) |
��3����KMnO4��Һ��Ũ��Ϊc�����ݹ�ϵʽ2KMnO4��5H2C2O4���㣻
��4������ϡ�����ữ��MnO4-����ԭΪMnO2��˫��ˮ�������������������ݵ���غ��֪���������������ɣ��ٸ���ԭ���غ��ж��Ƿ���ˮ���ɣ���ƽ��д��
����⣺��1�����������Һ����ǿ�����ԣ��ܹ�������ʽ�ζ��ܵ��ܣ�����Ӧ��ʹ����ʽ�ζ���ʢװ���������Һ���ʴ�Ϊ����ʽ��
��2��A��ʢװKMnO4��Һ�ĵζ�����ˮϴ��δ��KMnO4��Һ��ϴ�����������Һ��ϡ�ͣ�������ص����ʵ���ƫС�����²�����Һ�����ƫС���ⶨ�ĸ�����ص�Ũ��ƫ�ͣ���A���ϣ�
B��ʢ������Һ�ĵζ��ܵζ�ǰ���첿�������ݣ��ζ��յ�ʱ�����ݣ����²�����Һ�����ƫ�ⶨ�ĸ�����ص�Ũ��ƫ��B�����ϣ�
C���ζ�ʱ��������Һ����ƿ�⣬���²�����Һ�����ƫ�ⶨ�ĸ�����ص�Ũ��ƫ��C�����ϣ�
D����ƿ������ˮϴ����û�и���Բ�������ʵ���û��Ӱ�죬�ʶԲⶨ�ĸ�����ص�Ũ����Ӱ�죬��D�����ϣ�
��ѡ��A��
��3��n��H2C2O4��=0.100 mol/L��0.025L=0.0025mol��KMnO4��Һ��Ũ��Ϊc����
2KMnO4 ��5H2C2O4
2mol 5mol
c��0.02L 0.00025mol
����
=
�����c=0.05mol/L
�ʴ�Ϊ��0.05mol/L��
��4������ϡ�����ữ��MnO4-����ԭΪMnO2��MnԪ�ع�����3�ۣ�˫��ˮ������������������Ԫ�ع�����2�ۣ����ϼ���С������Ϊ6����MnO4-��ϵ��Ϊ2��MnO2��ϵ��Ϊ2��H2O2��ϵ��Ϊ3��O2ϵ��Ϊ3�����ݵ���غ��֪��OH-���ɣ���ϵ��Ϊ2����ԭ���غ��֪����ˮ���ɣ���ϵ��Ϊ2����Ӧ���ӷ���ʽΪ��2MnO4-+3H2O2=2MnO2��+3O2��+2OH-+2H2O��
�ʴ�Ϊ��2MnO4-+3H2O2=2MnO2��+3O2��+2OH-+2H2O��
��2��A��ʢװKMnO4��Һ�ĵζ�����ˮϴ��δ��KMnO4��Һ��ϴ�����������Һ��ϡ�ͣ�������ص����ʵ���ƫС�����²�����Һ�����ƫС���ⶨ�ĸ�����ص�Ũ��ƫ�ͣ���A���ϣ�
B��ʢ������Һ�ĵζ��ܵζ�ǰ���첿�������ݣ��ζ��յ�ʱ�����ݣ����²�����Һ�����ƫ�ⶨ�ĸ�����ص�Ũ��ƫ��B�����ϣ�
C���ζ�ʱ��������Һ����ƿ�⣬���²�����Һ�����ƫ�ⶨ�ĸ�����ص�Ũ��ƫ��C�����ϣ�
D����ƿ������ˮϴ����û�и���Բ�������ʵ���û��Ӱ�죬�ʶԲⶨ�ĸ�����ص�Ũ����Ӱ�죬��D�����ϣ�
��ѡ��A��
��3��n��H2C2O4��=0.100 mol/L��0.025L=0.0025mol��KMnO4��Һ��Ũ��Ϊc����
2KMnO4 ��5H2C2O4
2mol 5mol
c��0.02L 0.00025mol
����
| 2mol |
| 5mol |
| c��0.02L |
| 0.00025mol |
�ʴ�Ϊ��0.05mol/L��
��4������ϡ�����ữ��MnO4-����ԭΪMnO2��MnԪ�ع�����3�ۣ�˫��ˮ������������������Ԫ�ع�����2�ۣ����ϼ���С������Ϊ6����MnO4-��ϵ��Ϊ2��MnO2��ϵ��Ϊ2��H2O2��ϵ��Ϊ3��O2ϵ��Ϊ3�����ݵ���غ��֪��OH-���ɣ���ϵ��Ϊ2����ԭ���غ��֪����ˮ���ɣ���ϵ��Ϊ2����Ӧ���ӷ���ʽΪ��2MnO4-+3H2O2=2MnO2��+3O2��+2OH-+2H2O��
�ʴ�Ϊ��2MnO4-+3H2O2=2MnO2��+3O2��+2OH-+2H2O��
������������Ҫ����������ԭ��Ӧ��ƽ��������ԭ��Ӧ�ζ�Ӧ�á���ѧ����ȣ��Ѷ��еȣ�ע�����ʵ���ԭ������Ҫѧ���߱�һ�������۷��������ͼ����������������

��ϰ��ϵ�д�
�����Ŀ
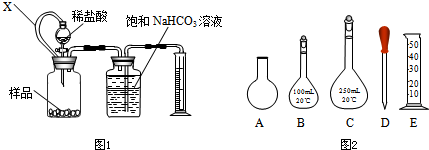
 ij��ѧѧϰС���������װ�ã��г�װ�����ԣ�������Լ����Բ������� ��O2������ٷ�����
ij��ѧѧϰС���������װ�ã��г�װ�����ԣ�������Լ����Բ������� ��O2������ٷ�����