��Ŀ����
����Ŀ��Ϊ�ⶨij�л�������A�Ľṹ����������ʵ�飺
(һ)����ʽ��ȷ����
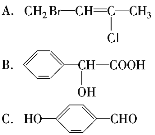
(1)���л���A�����������г��ȼ�գ�ʵ���ã�����5.4 g H2O��8.8 g CO2����������6.72 L(��״����)����������и�Ԫ�ص�ԭ�Ӹ�������_________________��

(2)�������Dzⶨ���л����������Է����������õ���ͼ����ʾ����ͼ��������Է�������Ϊ________�������ʵķ���ʽ��________��
(3)���ݼۼ����ۣ�Ԥ��A�Ŀ��ܽṹ��д���ṹ��ʽ________��
(��)�ṹʽ��ȷ����
(4)�˴Ź��������ܶ��л�������в�ͬλ�õ���ԭ�Ӹ�����ͬ�ķ�ֵ(�ź�)�����ݷ�ֵ(�ź�)����ȷ����������ԭ�ӵ��������Ŀ�����磺���ȼ���(Cl��CH2��O��CH3)��������ԭ����ͼ�ڡ����ⶨ���л���A�ĺ˴Ź�������ʾ��ͼ��ͼ�ۣ���A�Ľṹ��ʽΪ__________________________��

���𰸡�(1)N(C)��N(H) ��N(O)��2��6��1
(2)46 C2H6O (3)CH3CH2OH��CH3��O��CH3
(4)CH3CH2OH
��������(1)����������n(H2O)��0.3 mol������n(H)��0.6 mol��n(CO2)��0.2 mol������n(C)��0.2 mol������ԭ���غ���n(O)��n(H2O)��2n(CO2)��2n(O2)��0.3 mol��2��0.2 mol��2��![]() ��0.1 mol��������ʸ�Ԫ��ԭ�Ӹ�����N(C)��N(H)��N(O)��n(C)��n(H) ��n(O)��2��6��1��(2)��(1)��֪���л����ʵ��ʽΪC2H6O��������л���ķ���ʽΪ(C2H6O)m��������ͼ֪����Է�������Ϊ46����46m��46����m��1���������ʽΪC2H6O��(3)��A�ķ���ʽΪC2H6O��֪AΪ���ͻ�������Ʋ���ṹΪCH3CH2OH��CH3OCH3��(4)����A�ĺ˴Ź�������ͼ��֪��A�����ֲ�ͬ���͵�Hԭ�ӣ�CH3OCH3ֻ��һ�����͵�Hԭ�ӣ���A�Ľṹ��ʽΪCH3CH2OH��
��0.1 mol��������ʸ�Ԫ��ԭ�Ӹ�����N(C)��N(H)��N(O)��n(C)��n(H) ��n(O)��2��6��1��(2)��(1)��֪���л����ʵ��ʽΪC2H6O��������л���ķ���ʽΪ(C2H6O)m��������ͼ֪����Է�������Ϊ46����46m��46����m��1���������ʽΪC2H6O��(3)��A�ķ���ʽΪC2H6O��֪AΪ���ͻ�������Ʋ���ṹΪCH3CH2OH��CH3OCH3��(4)����A�ĺ˴Ź�������ͼ��֪��A�����ֲ�ͬ���͵�Hԭ�ӣ�CH3OCH3ֻ��һ�����͵�Hԭ�ӣ���A�Ľṹ��ʽΪCH3CH2OH��