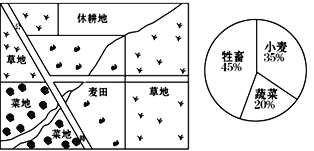
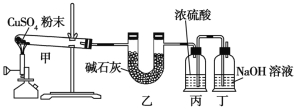
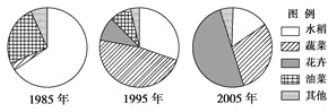
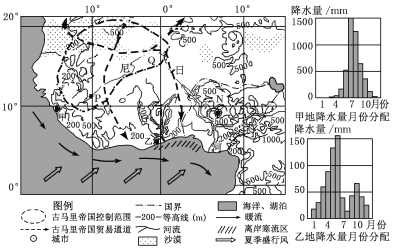
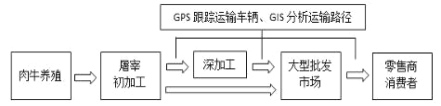
��Ŀ����
����Ŀ���밴Ҫ����գ�
(1)2 mol CO(NH2)2�к�________mol H��________g N��������ԭ�Ӹ�________mol H2O������ԭ�Ӹ�����ȡ�
(2)�ٱ�״���£�22.4 L CH4����1.5 mol NH3����1.806��1024��H2O���ӣ�
�ܱ�״���£�73 g HCl��������ԭ�Ӹ����ɶൽ�ٵ�˳����______________��
(3)2.3 g Na�к�________mol e-����������ˮ��Ӧ�в�����״���µ�H2________L��
(4)��0.4 mol Al2(SO4)3����Һ�У���________mol S![]() ��Al3+���ʵ���__________(�>����<����=��)0.8 mol��
��Al3+���ʵ���__________(�>����<����=��)0.8 mol��
���𰸡�(1)8 56 2 (2)��>��>��>�� (3)1.1 1.12 (4)1.2 <
��������(1)2 mol CO(NH2)2�к�8 mol H��4 mol N��2 mol O����Ԫ�ص�����Ϊ56 g��
(2)�ٱ�״���£�22.4 L CH4���ʵ���Ϊ1 mol������ԭ��4 mol����1.5 mol NH3����ԭ��4.5 mol����1.806��1024��H2O�������ʵ���Ϊ3 mol������ԭ��6 mol���ܱ�״���£�73 g HCl���ʵ���Ϊ2 mol������ԭ��2 mol�����Т�>��>��>�ܡ�
(3)n(Na)=![]() =0.1 mol������1����ԭ���к���11�����ӣ���2.3 g Na�к��е��ӵ����ʵ���n(e-)=11n(Na)=1.1 mol��
=0.1 mol������1����ԭ���к���11�����ӣ���2.3 g Na�к��е��ӵ����ʵ���n(e-)=11n(Na)=1.1 mol��
2Na+2H2O2NaOH+H2��
2 mol 22.4 L
0.1 mol x
2 mol��0.1 mol=22.4 L��x
x=![]() =1.12 L��
=1.12 L��
(4)��Al2(SO4)32Al3++3SO42-֪��
n(SO42��)=3n[Al2(SO4)3]=3��0.4 mol=1.2 mol��0.4 mol Al2(SO4)3�к���0.8 mol Al3+����ΪAl3+����Һ��ˮ�⣬��С��0.8 mol��

 ״Ԫ��ȫ��ͻ�Ƶ�����ϵ�д�
״Ԫ��ȫ��ͻ�Ƶ�����ϵ�д� ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�
ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�