��Ŀ����
����Ŀ��������һ�ּ��߷�չDZ���������Դ����̫����Ϊ�������Ȼ�ѧ���ѭ���ֽ�ˮ��һ�ָ�Ч������Ⱦ�����ⷽ�����䷴Ӧ��������ͼ��ʾ��
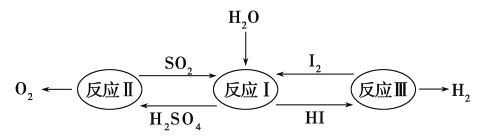
(1)��Ӧ��Ļ�ѧ����ʽ��________________��
(2)��Ӧ��õ��IJ�����I2���з��롣�ò������Һ�ڹ���I2�Ĵ����»�ֳ����㡪������Ũ��I2��H2SO4��ͺ���Ũ��I2��HI�㡣
������������ʵ������˵����ȷ����________(ѡ�����)��
a��������Һ���ܶȴ��ڲ���
b����I2ǰ��H2SO4��Һ��HI��Һ������
c��I2��HI��Һ�б���H2SO4��Һ������
�����������Һ�ķ�����____________________��
���������H2SO4����c(H��)��c(SO![]() )��2.06��1�����ֵ����2��ԭ��______________________��
)��2.06��1�����ֵ����2��ԭ��______________________��
(3)��Ӧ��
2H2SO4(l)===2SO2(g)��O2��2H2O(g)
��H����550 kJ��mol��1
����������Ӧ��ɣ�
��.H2SO4(l)===SO3(g)��H2O(g)
��H����177 kJ��mol��1
��SO3(g)�ֽ⡣
L(L1��L2)��X�ɷֱ����ѹǿ���¶�����ͼ��ʾLһ��ʱ������SO3(g)��ƽ��ת������X�ı仯��ϵ��
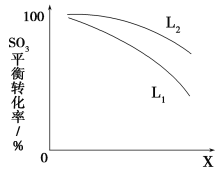
��X��������������________��
���ж�L1��L2�Ĵ�С��ϵ�����������ɣ�________________________________��
���𰸡�(1)SO2��I2��2H2O===H2SO4��2HI
(2)��ac �ڹ۲���ɫ����ɫ�����HI������ɫdz����H2SO4�� ��H2SO4�㺬������HI
(3) ��ѹǿ ��L1��L2 SO3(g)�ֽ���Ȼ�ѧ����ʽΪ2SO3(g) ![]() 2SO2(g)��O2(g) ��H����196 kJ��mol��1����ѹǿһ��ʱ���¶�������ƽ��ת��������
2SO2(g)��O2(g) ��H����196 kJ��mol��1����ѹǿһ��ʱ���¶�������ƽ��ת��������
��������(1)�ɷ�Ӧ����ͼʾ��֪����Ӧ��ķ�Ӧ��ΪSO2��I2��H2O��������ΪH2SO4��HI���Ӷ���д���÷�Ӧ�Ļ�ѧ����ʽ��(2)���������Ϣ���ò������Һ�ڹ��� I2�Ĵ����»�ֳ����㡪������Ũ�� I2�� H2SO4��ͺ���Ũ�� I2 �� HI �㡱�����ó������� I2 ǰ��Һδ���ֲַ�������I2 �������� HI ��Һ���ܽ�ȴ����� H2SO4��Һ���ܽ��������Һ���ܶȴ��ڲ��������ֲַ���������ac��ȷ��������HI��Һ�к���I2Ũ�ȴ�����Һ��ɫ������H2SO4��Һ�к���I2��Ũ�ȵ�����Һ��ɫdz����˿���ͨ���۲���Һ����ɫ����ɫ�����HI������ɫdz����H2SO4�㡣��H2SO4��ǿ���������ȫ�����ʹc(H��)��c(SO![]() )��2��1������Һ��c(H��)��c(SO
)��2��1������Һ��c(H��)��c(SO![]() )��2.06��1��˵����Һ��c(H��)������������ڸ�H2SO4���к�������ǿ��HI��HI�����H�����¡�(3)���ȸ��ݷ�Ӧ��ͷ�Ӧ��ȷ����Ӧ��Ϊ2SO3(g)
)��2.06��1��˵����Һ��c(H��)������������ڸ�H2SO4���к�������ǿ��HI��HI�����H�����¡�(3)���ȸ��ݷ�Ӧ��ͷ�Ӧ��ȷ����Ӧ��Ϊ2SO3(g) ![]() 2SO2(g)��O2(g) ��H����196 kJ��mol��1���ٸ��ݷ�Ӧ�����ص㣺����Ӧ����Ϊ��������������ȷ�Ӧ������ѹǿSO3(g)��ת���ʼ�С����X����ѹǿ�����ڵ�ѹ�������������¶�����Ӧ����ƽ���������ƶ���SO3(g)��ת������������L2������������������L1��L2��
2SO2(g)��O2(g) ��H����196 kJ��mol��1���ٸ��ݷ�Ӧ�����ص㣺����Ӧ����Ϊ��������������ȷ�Ӧ������ѹǿSO3(g)��ת���ʼ�С����X����ѹǿ�����ڵ�ѹ�������������¶�����Ӧ����ƽ���������ƶ���SO3(g)��ת������������L2������������������L1��L2��