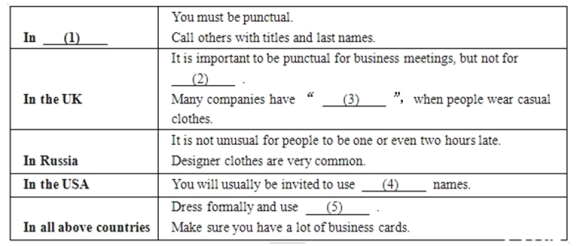
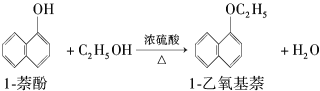
��Ŀ����
����Ŀ������NaHCO3������Һ����ø���Һ��pH���¶ȵı仯���£�
�¶�(��) | 10 | 20 | 30 | ������к���ȴ��50 �� |
pH | 8.3 | 8.4 | 8.5 | 8.8 |
��ͬѧ��Ϊ������Һ��pH���ߵ�ԭ����HCO![]() ��ˮ��̶����ʼ�����ǿ���÷�Ӧ�����ӷ���ʽΪ��________________��
��ˮ��̶����ʼ�����ǿ���÷�Ӧ�����ӷ���ʽΪ��________________��
��ͬѧ��ȼ���Ca(HCO3)2��Һ�������________������ΪNaHCO3��ҺpH���ߵ�ԭ����NaHCO3���ȷֽ⣬������Na2CO3�����ƶ�ˮ��̶ȣ�Na2CO3________NaHCO3(����ڡ���С�ڡ�)��
��ͬѧ��Ϊ�ס��ҵ��ж϶�����֡�����Ϊ��
(1)ֻҪ�ڼ�����к����Һ�м����������Լ�X����������������˵��________(��ס����ҡ�)�ж���ȷ���Լ�X��________(����ĸ)��
A��Ba(OH)2��Һ B��BaCl2��Һ
C��NaOH��Һ D�������ʯ��ˮ
(2)�����Ⱥ����Һ��ȴ��10 �棬����Һ��pH________(����ڡ��������ڡ����ڡ�)8.3����˵����һ�ж���ȷ��
���𰸡�HCO![]() ��H2O
��H2O![]() H2CO3��OH�� ���� ����
H2CO3��OH�� ���� ����
(1)�� B
(2)����
��������(1)HCO![]() ˮ�ⷽ��ʽ��HCO
ˮ�ⷽ��ʽ��HCO![]() ��H2O
��H2O![]() H2CO3��OH����
H2CO3��OH����
(2)������к���HCO![]() �ֽ��CO
�ֽ��CO![]() ������BaCl2��Һ�����ɳ���������ѡA��C��D����Ϊ���Ǿ���OH��������HCO
������BaCl2��Һ�����ɳ���������ѡA��C��D����Ϊ���Ǿ���OH��������HCO![]() ��Ӧ����CO
��Ӧ����CO![]() ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�