��Ŀ����
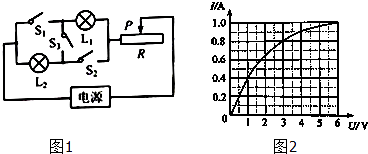
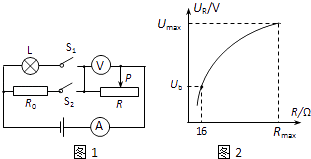
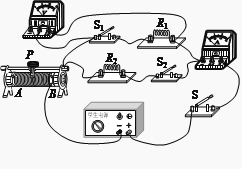
����Ŀ����ͼ��ʾ�ĵ�·�У���Դ���˵ĵ�ѹ���ֲ��䣬��ֵ�ֱ�ΪR1��R2�Ķ�ֵ��������ֵ�����¶ȱ仯�������������������ֵΪR3�����պϿ���S��S1 �� �Ͽ�����S2 �� �����������Ļ�ƬP�����е�ʱ����ѹ����ʾ��ΪU1 �� �����������ĵ繦��P1=2.4W����������ʾ��ΪI1�����պϿ���S��S2 �� �Ͽ�����S1 �� �����������Ļ�ƬP�����˵�Aʱ����ѹ����ʾ��ΪU2 �� ��·���ܵ繦��P2=2.88W����������ʾ��ΪI2�������п��ض��պϣ������������Ļ�ƬP�����˵�Bʱ����·���ܵ繦��Ϊ P3����֪��![]() ����
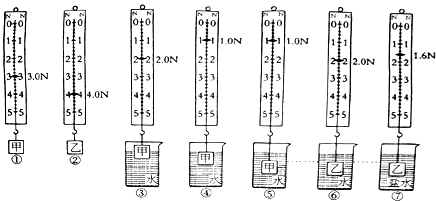
����
��1��R2��R3����ֵ֮�ȣ�
��2��U1��U2֮�ȣ�
��3���ܵ繦��P3��
���𰸡��⣺��1���������� ![]() =
=![]() ����
����![]() =
=![]()
������֪���ݽ�ã� ![]() =
=![]()
��2�����ڼס���������·�����ܵ�ѹ��ͬ�������� ![]() =
=![]() =
=![]()
��![]() =
=![]() ����ɽ�ã�
����ɽ�ã� ![]()
����ŷķ������ ![]()
��3�������ҡ���������·�����ܵ�ѹ��ͬ�������� 
��R2��R3��R1�Ĺ�ϵ���룬�ɽ�ã� ![]()
�����ҡ���������·�����ѹ��ͬ������ ![]()
���� ![]()
���������պϿ���S��S1 �� �Ͽ�����S2 �� �����������Ļ�ƬP�����е�ʱ����·��ͼ����ʾ���պϿ���S��S2 �� �Ͽ�����S1 �� �����������Ļ�ƬP�����˵�Aʱ����·��ͼ����ʾ�������п��ض��պϣ������������Ļ�ƬP�����˵�Bʱ����·��ͼ��7����ʾ��
�����㾫����������Ŀ����֪����������ŷķ���ɼ���Ӧ�ú͵繦�ʵļ��㹫ʽ�����֪ʶ���Եõ�����Ĵ𰸣���Ҫ����ŷķ���ɵ�Ӧ�ã� �� ͬһ�����裬��ֵ���䣬������͵�ѹ�� ����������������˵ĵ�ѹ����ʱ��ͨ���ĵ���Ҳ����R=U/I�� �� ����ѹ����ʱ������Խ����ͨ���ĵ�����ԽС����I=U/R�� �� ������һ��ʱ������Խ����������˵ĵ�ѹ��Խ��U=IR��������繦�ʹ�ʽ��P =W/t ��P=UI ; ʽ�е�λP����(w)��W������t���룻U������V����I������A������繦�ʻ����ù�ʽ��P=I2R��P= U2/R��

 �»����ܶ�Ա��ϵ�д�
�»����ܶ�Ա��ϵ�д� ����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�