��Ŀ����
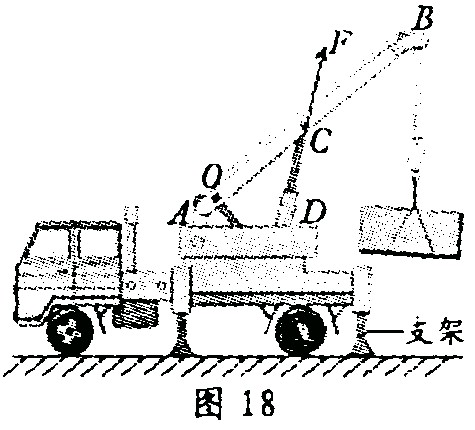
�ڿ�������У�Ϊ�˴ӿ����Ľ����о����ʵʩ��Ԯ��Ӫ��������ʹ�õ��˴������������ػ������ڼ��и�ϰ��С��ͬѧ���������ֳ��ľ�Ԯ������ڸж�֮�࣬������һ̨����������ػ���������̽����
С��Ҫ̽���������ǣ����ػ���������ᄇֹ�ڿ���ʱ��
�� ֧�ű۶����ر۵���������С���� ˮƽ�����ܵ������ػ���ѹǿ��
Ϊ�ˣ�С�³����ػ��⣬�������������ģ�������ɲ����ơ����ߡ�����㹻�������ֽ��īˮ��ë�ʡ���дֽ��
��1��Ҫ�������̽�������������л�Ӧ�����ӵ���________________��
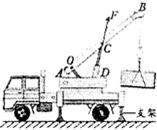
��2��Ϊ�������ٵ�̽������Ҫ�������ۡ�������ͼ������֧�ű�CD �����ر�AB ��������F�����ۣ�����O ����֧�㡣����ܰ��ʾ��ǧ���������ͼ�л�����Ӵ����
��3��������ڵ�̽���У���ԽӴ�����IJ��������������ѣ�С�����������ʵ�鷽����
����һ��ʵ�鲽��Ϊ��
A��������˩������õ��ɲ����Ʒֱ���������ػ�������G1�����������G2��
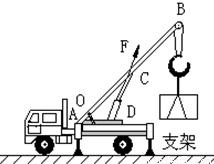
B��������������ػ��Ĺҹ��ϣ���ͼ��ʾ��
C����ˮƽ�����ϴ������������̷�����ֽ����дֽ��Ȼ�����ػ�ƽ���ڸ�дֽ�ϣ���������װ�ô��ھ�ֹ״̬��
D��ȡ�����ػ���������̥��֧��������ֽ�����µ�ӡ������������ػ���������̥��֧�ܣ���ˮƽ����ĽӴ����S��
E�����ˮƽ�����ܵ������ػ���ѹǿp��________________��
�������뷽��һֻ�в���C ��ͬ���������С������������ɲ���C
C����ˮƽ��������������ֽ��������ë�������ػ�����̥��֧����Ϳ��īˮ��Ȼ�����ػ�ƽ��������ֽ�ϣ���������װ�ô���__________״̬��
��4��ʵ�������С�¶�����ڵ�̽���нӴ�����IJ�����Ȼ�е��������⣬��Ϊ��������ȷ��������ѡһ�ַ���������ָ�����������ȷ��ԭ��_______________________________________��
��5����ʵ�ʾ����У�һ�������������ػ�����2��104N�����֧�ű�CD �����ر�AB ��������F��������0.8m�������������3.2m������F�Ĵ�С��
С��Ҫ̽���������ǣ����ػ���������ᄇֹ�ڿ���ʱ��
�� ֧�ű۶����ر۵���������С���� ˮƽ�����ܵ������ػ���ѹǿ��
Ϊ�ˣ�С�³����ػ��⣬�������������ģ�������ɲ����ơ����ߡ�����㹻�������ֽ��īˮ��ë�ʡ���дֽ��
��1��Ҫ�������̽�������������л�Ӧ�����ӵ���________________��
��2��Ϊ�������ٵ�̽������Ҫ�������ۡ�������ͼ������֧�ű�CD �����ر�AB ��������F�����ۣ�����O ����֧�㡣����ܰ��ʾ��ǧ���������ͼ�л�����Ӵ����
��3��������ڵ�̽���У���ԽӴ�����IJ��������������ѣ�С�����������ʵ�鷽����
����һ��ʵ�鲽��Ϊ��
A��������˩������õ��ɲ����Ʒֱ���������ػ�������G1�����������G2��
B��������������ػ��Ĺҹ��ϣ���ͼ��ʾ��
C����ˮƽ�����ϴ������������̷�����ֽ����дֽ��Ȼ�����ػ�ƽ���ڸ�дֽ�ϣ���������װ�ô��ھ�ֹ״̬��
D��ȡ�����ػ���������̥��֧��������ֽ�����µ�ӡ������������ػ���������̥��֧�ܣ���ˮƽ����ĽӴ����S��
E�����ˮƽ�����ܵ������ػ���ѹǿp��________________��
�������뷽��һֻ�в���C ��ͬ���������С������������ɲ���C
C����ˮƽ��������������ֽ��������ë�������ػ�����̥��֧����Ϳ��īˮ��Ȼ�����ػ�ƽ��������ֽ�ϣ���������װ�ô���__________״̬��
��4��ʵ�������С�¶�����ڵ�̽���нӴ�����IJ�����Ȼ�е��������⣬��Ϊ��������ȷ��������ѡһ�ַ���������ָ�����������ȷ��ԭ��_______________________________________��
��5����ʵ�ʾ����У�һ�������������ػ�����2��104N�����֧�ű�CD �����ر�AB ��������F��������0.8m�������������3.2m������F�Ĵ�С��

��1���̶ȳߣ������ǰ壩
��2��

��3����G1+G2��/S����ֹ
��4���ڷ���һ�У���������������ػ�������������ᣬ�������ڸ�дֽ�ϵ�ӡ�������������ڷ������У�����īˮͿĨ�����ȣ�����ӡ��̫�̫dz���߽�Ⱦ������ֽ�ϽӴ�������ĵط���
��5���⣺���ݸܸ�ƽ������
F��lF=G��lG
F��0.8m= G��3.2m
F=8��104N��
��4���ڷ���һ�У���������������ػ�������������ᣬ�������ڸ�дֽ�ϵ�ӡ�������������ڷ������У�����īˮͿĨ�����ȣ�����ӡ��̫�̫dz���߽�Ⱦ������ֽ�ϽӴ�������ĵط���
��5���⣺���ݸܸ�ƽ������
F��lF=G��lG
F��0.8m= G��3.2m
F=8��104N��

��ϰ��ϵ�д�
�����Ŀ
 �ڿ�������У�Ϊ�˴ӿ����Ľ����о����ʵʩ��Ԯ��Ӫ��������ʹ�õ��˴������������ػ������ڼ��и�ϰ��С��ͬѧ���������ֳ��ľ�Ԯ������ڸж�֮�࣬������һ̨����������ػ���������̽����
�ڿ�������У�Ϊ�˴ӿ����Ľ����о����ʵʩ��Ԯ��Ӫ��������ʹ�õ��˴������������ػ������ڼ��и�ϰ��С��ͬѧ���������ֳ��ľ�Ԯ������ڸж�֮�࣬������һ̨����������ػ���������̽����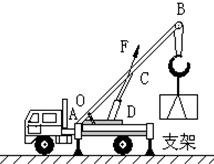 �ڿ�������У�Ϊ�˴ӿ����Ľ����о����ʵʩ��Ԯ��Ӫ��������ʹ�õ��˴������������ػ������ڼ��и�ϰ��С��ͬѧ���������ֳ��ľ�Ԯ������ڸж�֮�࣬������һ̨����������ػ���������̽����
�ڿ�������У�Ϊ�˴ӿ����Ľ����о����ʵʩ��Ԯ��Ӫ��������ʹ�õ��˴������������ػ������ڼ��и�ϰ��С��ͬѧ���������ֳ��ľ�Ԯ������ڸж�֮�࣬������һ̨����������ػ���������̽���� �ڿ�������У�Ϊ�˴ӿ����Ľ����о����ʵʩ��Ԯ��Ӫ��������ʹ�õ��˴������������ػ������ڼ��и�ϰ��С��ͬѧ���������ֳ��ľ�Ԯ������ڸж�֮�࣬������һ̨����������ػ���������̽����
�ڿ�������У�Ϊ�˴ӿ����Ľ����о����ʵʩ��Ԯ��Ӫ��������ʹ�õ��˴������������ػ������ڼ��и�ϰ��С��ͬѧ���������ֳ��ľ�Ԯ������ڸж�֮�࣬������һ̨����������ػ���������̽����