��Ŀ����
���ⶨ������ֵ��ʵ�飺
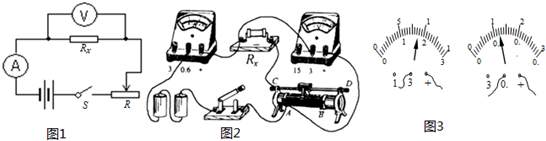
��1��С��������ͼ����ʾ�ĵ�·ͼ����ʵ���������ӳ�ʵ���·��ͼ�ң�ͬС���С���ڼ��ʱ��Ϊ����ʵ��Ŀ������ʵ���·����һ���������Ӵ��ˣ�����С���Ľӣ��������ڽӴ����Ǹ����ϴ�����������һ�����ߣ�ʹ��·������ȷ����������Ľ�������ߣ���ʵ���Ӱ���ǣ�
��2��������������ʵ���г����𱣻���·�������⣬��һ����Ҫ����
��3��С������·�Ľ���ȷ���Ͽ��أ����ڱ������Ļ�Ƭ��ijλ��ʱ����ѹ���͵�������ָʾ��ͼ����ʾ�����ѹ���Ķ�����
��1��С��������ͼ����ʾ�ĵ�·ͼ����ʵ���������ӳ�ʵ���·��ͼ�ң�ͬС���С���ڼ��ʱ��Ϊ����ʵ��Ŀ������ʵ���·����һ���������Ӵ��ˣ�����С���Ľӣ��������ڽӴ����Ǹ����ϴ�����������һ�����ߣ�ʹ��·������ȷ����������Ľ�������ߣ���ʵ���Ӱ���ǣ�
��ѹ�������IJ��Ǵ���������˵ĵ�ѹ
��ѹ�������IJ��Ǵ���������˵ĵ�ѹ
������˵����ʲôӰ�죩����2��������������ʵ���г����𱣻���·�������⣬��һ����Ҫ����
�ı�Rx���˵ĵ�ѹ����β�����ƽ��ֵ����Сʵ�����
�ı�Rx���˵ĵ�ѹ����β�����ƽ��ֵ����Сʵ�����
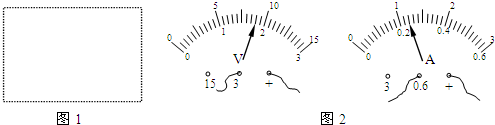
����3��С������·�Ľ���ȷ���Ͽ��أ����ڱ������Ļ�Ƭ��ijλ��ʱ����ѹ���͵�������ָʾ��ͼ����ʾ�����ѹ���Ķ�����
1.8
1.8
V���������Ķ�����0.24
0.24
A���������Rx����ֵ��7.5
7.5
����
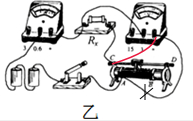
��������1�����ݣ��ף��е�·ͼ��˳�ŵ����ķ������ӣ��ң��е�ʵ��ͼ�����������·��������ѹ����������Rx���˵ĵ�ѹ�����ֵ�ѹ�������ӳ��������⣻
��2�������������ڵ�·�е������������棺������·���ı��õ������˵�ѹ��
��3�������ѹ�����������������ֶ�ֵ������ָ��ָʾ����ֵ����ѹ��������������Ҫ������
��2�������������ڵ�·�е������������棺������·���ı��õ������˵�ѹ��
��3�������ѹ�����������������ֶ�ֵ������ָ��ָʾ����ֵ����ѹ��������������Ҫ������
����⣺
��1�����ݵ�·ͼ����ʵ��ͼ�����ֵ�ѹ������ǻ�����������Rx���˵ĵ�ѹ����Ȼ��ʵ��Ҫ����������ʹRx���˵ĵ�ѹƫ����Rx����ֵƫ������ͼ��ʾ��
��2��Ҫ����������ֵ����Ҫ��β���ȡƽ��ֵ��������Ҫ�ı们����������Ƭλ�ã��ı����������˵�ѹ��ͨ���ĵ������õ���ͬ�ĵ���ֵȡ��ƽ��ֵ��Ϊ���������
��3����ͼ֪����ѹ������������0��3V���ֶ�ֵΪ0.1V������������������0��0.6A���ֶ�ֵ��0.02A�����Ե�ѹU=1.8V������I=0.24A��
����ŷķ���ɵã�Rx=
=
=7.5����
�ʴ�Ϊ��
��2���ı�Rx���˵ĵ�ѹ����β�����ƽ��ֵ����Сʵ����
��3��1.8��0.24��7.5��
��1�����ݵ�·ͼ����ʵ��ͼ�����ֵ�ѹ������ǻ�����������Rx���˵ĵ�ѹ����Ȼ��ʵ��Ҫ����������ʹRx���˵ĵ�ѹƫ����Rx����ֵƫ������ͼ��ʾ��
��2��Ҫ����������ֵ����Ҫ��β���ȡƽ��ֵ��������Ҫ�ı们����������Ƭλ�ã��ı����������˵�ѹ��ͨ���ĵ������õ���ͬ�ĵ���ֵȡ��ƽ��ֵ��Ϊ���������
��3����ͼ֪����ѹ������������0��3V���ֶ�ֵΪ0.1V������������������0��0.6A���ֶ�ֵ��0.02A�����Ե�ѹU=1.8V������I=0.24A��
����ŷķ���ɵã�Rx=
| U |
| I |
| 1.8V |
| 0.24A |
�ʴ�Ϊ��

��2���ı�Rx���˵ĵ�ѹ����β�����ƽ��ֵ����Сʵ����
��3��1.8��0.24��7.5��
���������⿼�����÷�������������ֵ���������漰���˵�·�����ӡ����������ѹ���Ķ�����������ԭ�������ۺ���ǿ�������ŷķ��������������⣮

��ϰ��ϵ�д�
�����Ŀ