��Ŀ����
 С��Ϊ�Ƚϡ���ͬ���ʵ�������������������µ�ʵ�鷽������������ȵ�ˮ��ú�ͷֱ�װ���ձ��У��̶�������̨�ϣ���������ͬ�ľƾ���ͬʱ����ˮ��ú�ͣ�ʵ��װ����ͼ��ʾ��ʵ��ʱÿ��һ��ʱ��ͬʱ����ˮ��ú�͵��¶ȣ�
С��Ϊ�Ƚϡ���ͬ���ʵ�������������������µ�ʵ�鷽������������ȵ�ˮ��ú�ͷֱ�װ���ձ��У��̶�������̨�ϣ���������ͬ�ľƾ���ͬʱ����ˮ��ú�ͣ�ʵ��װ����ͼ��ʾ��ʵ��ʱÿ��һ��ʱ��ͬʱ����ˮ��ú�͵��¶ȣ���1���ڰ�װ������ʵ������ʱ����ѧ������˳���ǣ���ͼ�У����ȵ����̶�
B
B
��λ�ã��ٵ����̶�A
A
��λ�ã�ѡ�A����B��������2���±���С����̽�������м�¼�����ݣ��ɱ����֪��С����ͨ���Ƚ�
����ʱ��
����ʱ��
�����Ƚ�ˮ��ú�͵����������ģ��ɱ�����С���ó��Ľ�����ˮ������������ú�ʹ�
ˮ������������ú�ʹ�
��| ���� | ���� | ���� | ���ߵ��¶� | ����ʱ�� |
| ˮ | 1 | 0.1 | 10 | 2 |
| 2 | 0.2 | 10 | 4 | |
| ú�� | 3 | 0.3 | 10 | 1 |
| 4 | 0.4 | 10 | 2 |
��������1�����þƾ��Ƽ���ʱ�����Ƕ������þƾ��Ƶ�������ȵģ��ʾݴ����ж��ȵ����̶���һװ�ã�������̶���һװ�ã�
��2������Ŀ�зֱ�����ͬ�ľƾ��Ƽ��ȣ���˵������ͬ��ʱ�䣬��ͬ�ľƾ������ų�����������ͬ �ģ�����Ƚϲ�ͬ���ʵ��������������Ƚ�������ͬ�IJ�ͬ���ʣ���������ͬ�¶�ʱ�����õļ���ʱ��ij��̼��ɣ�����ʱ�䳤����������ǿ��
��2������Ŀ�зֱ�����ͬ�ľƾ��Ƽ��ȣ���˵������ͬ��ʱ�䣬��ͬ�ľƾ������ų�����������ͬ �ģ�����Ƚϲ�ͬ���ʵ��������������Ƚ�������ͬ�IJ�ͬ���ʣ���������ͬ�¶�ʱ�����õļ���ʱ��ij��̼��ɣ�����ʱ�䳤����������ǿ��
����⣺��1�����ھƾ��Ƶ������¶���ߣ�����������Ҫ�����ձ����ƾ�������ĸ߶ȣ����þƾ���������ȣ��ڵ����¶ȼ���ˮ�е�λ�ã���ʵ��ǰ�ȵ����̶�B��λ�ã��ٵ����̶�A��λ�ã�
��2������ʹ�õ�����ͬ�ľƾ��ƣ���ʾ����ͬ��ʱ���ڷų���������ȣ����������յ�������ȣ��ʼ���ʱ����Լ�ӷ�ӳˮ��ú�����������Ķ��٣�
����������ͬ������ˮ��ú�ͣ�����������������ͬ�¶�ʱ��ˮ��Ҫ��ʱ�䳤����ˮ����������Ҫ��һЩ��
�ʴ�Ϊ����1��B��A����2������ʱ�䣻ˮ������������ú�ʹ�
��2������ʹ�õ�����ͬ�ľƾ��ƣ���ʾ����ͬ��ʱ���ڷų���������ȣ����������յ�������ȣ��ʼ���ʱ����Լ�ӷ�ӳˮ��ú�����������Ķ��٣�
����������ͬ������ˮ��ú�ͣ�����������������ͬ�¶�ʱ��ˮ��Ҫ��ʱ�䳤����ˮ����������Ҫ��һЩ��
�ʴ�Ϊ����1��B��A����2������ʱ�䣻ˮ������������ú�ʹ�
������������˼����������ת�����Ϳ��Ʊ�������˼�룬��Ϊ����ע�����ã�

��ϰ��ϵ�д�
�����Ŀ
 ��2013?������С��Ϊ�Ƚϡ���ͬ���ʵ�������������������µ�ʵ�鷽������������ͬ��ˮ��ú�ͷֱ�װ���ձ��У��̶�������̨�ϣ���������ͬ�ľƾ���ͬʱ����ˮ��ú�ͣ�ʵ��װ����ͼ��ʾ��ʵ��ʱÿ��һ��ʱ��ͬʱ����ˮ��ú�͵��¶ȣ�
��2013?������С��Ϊ�Ƚϡ���ͬ���ʵ�������������������µ�ʵ�鷽������������ͬ��ˮ��ú�ͷֱ�װ���ձ��У��̶�������̨�ϣ���������ͬ�ľƾ���ͬʱ����ˮ��ú�ͣ�ʵ��װ����ͼ��ʾ��ʵ��ʱÿ��һ��ʱ��ͬʱ����ˮ��ú�͵��¶ȣ�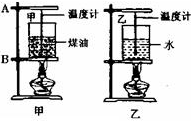 ��2010?ͨ����ģ�⣩С��Ϊ�Ƚϡ���ͬ���ʵ�������������������µ�ʵ�鷽������������ȵ�ˮ��ú�ͷֱ�װ���ձ��У��̶�������̨�ϣ���������ͬ�ľƾ���ͬʱ����ˮ��ú�ͣ�ʵ��װ����ͼ��ʾ��ʵ��ʱÿ��һ��ʱ��ͬʱ����ˮ��ú�͵��¶ȣ�
��2010?ͨ����ģ�⣩С��Ϊ�Ƚϡ���ͬ���ʵ�������������������µ�ʵ�鷽������������ȵ�ˮ��ú�ͷֱ�װ���ձ��У��̶�������̨�ϣ���������ͬ�ľƾ���ͬʱ����ˮ��ú�ͣ�ʵ��װ����ͼ��ʾ��ʵ��ʱÿ��һ��ʱ��ͬʱ����ˮ��ú�͵��¶ȣ�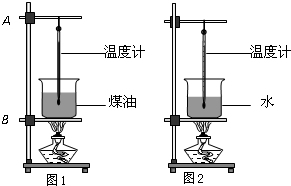 С��Ϊ�Ƚϡ���ͬ���ʵ�������������������µ�ʵ�鷽������������ͬ��ˮ��ú�ͷֱ�װ���ձ��У��̶�������̨�ϣ���������ͬ�ľƾ���ͬʱ����ˮ��ú�ͣ�ʵ��װ����ͼ��ʾ��ʵ��ʱÿ��һ��ʱ��ͬʱ����ˮ��ú�͵��¶ȣ�
С��Ϊ�Ƚϡ���ͬ���ʵ�������������������µ�ʵ�鷽������������ͬ��ˮ��ú�ͷֱ�װ���ձ��У��̶�������̨�ϣ���������ͬ�ľƾ���ͬʱ����ˮ��ú�ͣ�ʵ��װ����ͼ��ʾ��ʵ��ʱÿ��һ��ʱ��ͬʱ����ˮ��ú�͵��¶ȣ� С��Ϊ�Ƚϡ���ͬ���ʵ�������������������µ�ʵ�鷽������ˮ��ú�ͷֱ�װ���ձ��У��̶�������̨�ϣ���������ͬ�ľƾ��Ƽ���ˮ��ú�ͣ�ʵ��װ����ͼ��
С��Ϊ�Ƚϡ���ͬ���ʵ�������������������µ�ʵ�鷽������ˮ��ú�ͷֱ�װ���ձ��У��̶�������̨�ϣ���������ͬ�ľƾ��Ƽ���ˮ��ú�ͣ�ʵ��װ����ͼ��