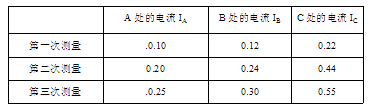
��Ŀ����
����Ŀ����̽�������������Ĺ�ϵ��ʵ���У���Ҫ�����������⣺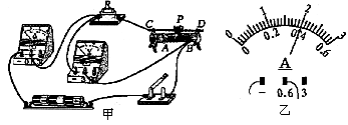
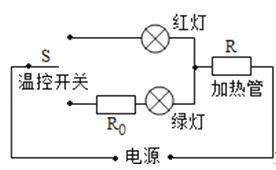
��1��С�½�ʵ�����ӳ���ͼ����ʾ��·��������һ�����ߴ������������Ӵ���ĵ����ϻ������������� .
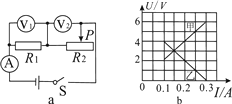
��2������·������ȷ�պϿ��أ��ƶ�������������ƬP��ʹ��ֵ����R���˵�ѹΪ2V��������ʾ����ͼ����ʾ��������СΪ A .
��3����5�����軻��10���ĵ���պϿ��أ���ѹ��ʾ���� ��ѡ����С��������ʱӦ����ƬP�� (ѡ�A"��B��)���ƶ���ʹ�������˵�ѹΪ V .
��4��ͨ����ʵ�飬���Եó����ۣ�
���𰸡�
��1���⣺��ͼ��ʾ��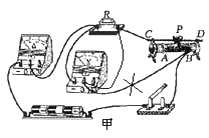
��2��0.4
��3�����A��2
��4������ѹһ��ʱ��ͨ������ĵ����뵼��ĵ���ɷ���
����������1����ͼ֪����ѹ������ǵ���ͻ������������ܵ�ѹ��Ӧ����������������貢������ͼ��ʾ�� ��
��
��2����ͼ��֪��������������Ϊ00.6A���ֶ�ֵΪ0.02A������ʾ��Ϊ0.4A��
��3����(2)֪��ԭ�������ֵR ![]() 5����������10���ĵ�����ݴ���ѹ���ص㣬����ֵõĵ�ѹ���ѹ����ʾ����������ڻ������������������ұߵ�B������������Ӧ�ý���Ƭ����˵�A�ƶ�����������������ֵ������ֵõĵ�ѹ��ʹ�������˵ĵ�ѹ��Ϊ2V��
5����������10���ĵ�����ݴ���ѹ���ص㣬����ֵõĵ�ѹ���ѹ����ʾ����������ڻ������������������ұߵ�B������������Ӧ�ý���Ƭ����˵�A�ƶ�����������������ֵ������ֵõĵ�ѹ��ʹ�������˵ĵ�ѹ��Ϊ2V��
��4��ͨ����ʵ�飬���Եó����ۣ�����ѹһ��ʱ�������еĵ��������ĵ���ɷ��� .
���Դ��ǣ���1������𣻣�2��0.4����3�����A��2����4������ѹһ��ʱ��ͨ������ĵ����뵼��ĵ���ɷ��� .