��Ŀ����
����Ŀ��С��ͬѧ��A��B�����塢���롢��ĭ������̽����ѹ��������Ч����ʲô�����йء�ʵ�顣
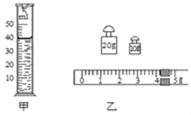
��1��ʵ����С����ͨ���۲�___���Ƚ�ѹ������Ч���ġ�
��2���Ƚϼס�����ͼ�ܹ��õ��Ľ�����_______________��
��3����Ҫ̽����ѹ��������Ч�������������С�Ĺ�ϵ����Ӧͨ���Ƚ�ͼ___��ʾʵ�顣��ʱʵ����Ҫ���ƣ�_______���䣻
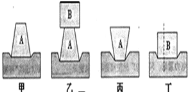
��4��С��ͬѧʵ��ʱ������B����ֱ�����гɴ�С��ͬ�����飬��ͼ����ʾ�����������Ƕ���ĭ��ѹ������Ч����ͬ���ɴ����ó��Ľ����ǣ�ѹ������Ч������������ء�����Ϊ����̽�������д��ڵ�������______________��
���𰸡���ĭ�İ��ݳ̶� �������һ��ʱ��ѹ��Խ��ѹ������Ч��Խ���� �ͱ� ѹ�� û�п���ѹ������
��������
��1��ʵ���У���ĭ�İ��ݳ̶�Խ��ѹ������Ч��Խ���ԣ�������ĭ�İ��ݳ̶�����ӳѹ��������Ч����
��2���ס�����ʵ���У����������ͬ��ѹ��Խ����ĭ����Խ�ʿɵý��ۣ��������һ��ʱ��ѹ��Խ��ѹ������Ч��Խ���ԣ�
��3��Ҫ�ó�ѹ��������Ч�������������С�Ĺ�ϵ����Ҫ����ѹ�����䣬�ı�����������ͱ���ͼ�������⣻
��4���о�ѹ������Ч������������Ĺ�ϵʱ��Ӧ�ñ���ѹ�����䣬С��ʵ��ʱû�б���ѹ�����䣬ʵ�������ѹ�����������ͬʱ�仯�����о�ѹ������Ч������������Ĺ�ϵ��

����Ŀ����ʵ����������У�����ͬѧ�鵽��̽��������·��ѹ�Ĺ��ɡ���ʵ�飬��ʵ��������£�
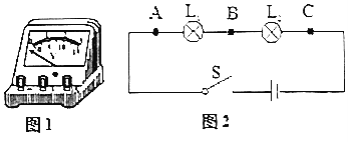
��1�����ڼ��ʵ������ʱ���ֵ�ѹ����ָ��λ����ͼ��ʾ����������Ҫ�Ե�ѹ�����еIJ�����________��
��2����������ʼ����ͼ���Ӻõ�·���ÿ����Դ�ʱ���ֵ�ѹ����ָ����ƫת����ͼ��λ�ã����ѹ�������������ԭ����________��
��3��������������õ�ѹ���ֱ����L1���˵ĵ�ѹUAB��L2���˵ĵ�ѹUBC��L1��L2���˵��ܵ�ѹUAC�� ������ͬ���ݣ���������ʵ�飬�����β���ĵ�ѹ�����±���
ʵ����� | UAB | UBC | UAC |
��һ�� | 0.8 | 2.2 | 3 |
�ڶ��� | 1.0 | 2.0 | 3 |
������ | 1.2 | 1.8 | 3 |
�����������ʵ����̺��й����ݻش��������⣮
��������Ƶı����д��ڵIJ���֮����________��
�ڷ��������е����ݣ��ó�ʵ�������________��
��������ʵ���ж���Ҫ���ж�β������е���Ϊ���ҵ��ձ���ɣ�����żȻ�ԣ��е���Ϊ����ƽ��ֵ�Լ�С������ʵ���ж�β�����Ŀ���뱾ʵ����ͬ����________������ĸ����
A����������ij��� B������������� C��̽��������·�е������ɣ�