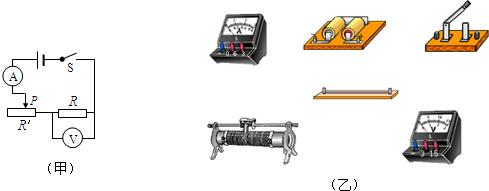
��Ŀ����
Ϊ��̽����������ѹ�Ĺ�ϵ��С�������ͼ����ʾ�ĵ�·ͼ��

��1������ݵ�·ͼ���ñʻ��ߴ��浼�߽�ͼ�ҵ�Ԫ����������������ѹ���������������̼�ͼ����
��2�������ӵ�·ͼʱ������Ӧ��ʼ����
��3��С��ͬѧ�ڱպϿ���S���ֵ�ѹ����ʾ������������û��ʾ������ɴ������ԭ������ǵ���R
��4��С���Աߵ�һ��ͬѧ�����·���֣����ػ�û�պϣ���ѹ��ʾ�����������رպϺ��ѹ����ʾ����СΪ�㣬������ʾ�����û�г������̣�����Ϊ����ͬѧ���ߵĴ�����
��5��С��ͬѧ�ų����Ϻ�ʼʵ�飬�����ڻ�Ƭλ�ã��ı䶨ֵ�������˵ĵ�ѹ��ǰ���ε�ѹ������������ʾ�������һ������ʾ����3�����������ʾ����ͼ����ʾ��
����ѵ�3�εĵ���������ڱ���һ���У�
���ɱ���һ�������ݿ�֪ʵ��ʹ�õĶ�ֵ�������ֵ��
�۶Ա���һ�������ݽ��з������ɹ��ɳ��Ľ�����
����һ��
��������
��6���������ͼ��·̽�����������Ĺ�ϵ��ʵ���¼���������������ʾ��
�ٸ��ݱ����������ݿɵó�������
�����о�����������ϵʱ����֤��ֵ�������˵�ѹ3V���䣬����5���Ķ�ֵ�������ʵ�飬�ٻ���10���Ķ�ֵ����ʱ��ijͬѧû�иı们����������Ƭ��λ�ã���ô�����Ͽ��غ�ѹ����ʾ����

��1������ݵ�·ͼ���ñʻ��ߴ��浼�߽�ͼ�ҵ�Ԫ����������������ѹ���������������̼�ͼ����
��2�������ӵ�·ͼʱ������Ӧ��ʼ����
�Ͽ�
�Ͽ�
�ģ�����Ͽ������պϡ������պϿ���ǰ����Ƭ��Ӧ���ڱ����������·�е���ֵ���
���
��������С����������3��С��ͬѧ�ڱպϿ���S���ֵ�ѹ����ʾ������������û��ʾ������ɴ������ԭ������ǵ���R
��·
��·
����ѡ���·����·������4��С���Աߵ�һ��ͬѧ�����·���֣����ػ�û�պϣ���ѹ��ʾ�����������رպϺ��ѹ����ʾ����СΪ�㣬������ʾ�����û�г������̣�����Ϊ����ͬѧ���ߵĴ�����
�ѿ���S�����ڵ��������
�ѿ���S�����ڵ��������
����5��С��ͬѧ�ų����Ϻ�ʼʵ�飬�����ڻ�Ƭλ�ã��ı䶨ֵ�������˵ĵ�ѹ��ǰ���ε�ѹ������������ʾ�������һ������ʾ����3�����������ʾ����ͼ����ʾ��
����ѵ�3�εĵ���������ڱ���һ���У�
���ɱ���һ�������ݿ�֪ʵ��ʹ�õĶ�ֵ�������ֵ��
20��
20��
���۶Ա���һ�������ݽ��з������ɹ��ɳ��Ľ�����
��ѹ���䣬�����еĵ��������ĵ���ɷ���
��ѹ���䣬�����еĵ��������ĵ���ɷ���
������һ��
| ���� | 1 | 2 | 3 |
| ��ѹ��V�� | 2.0 | 4.0 | |
| ������A�� | 0.1 | 0.2 |
| ���� | 1 | 2 | 3 |
| ���裨���� | 5 | 10 | 15 |
| ������A�� | 0.6 | 0.3 | 0.2 |
�ٸ��ݱ����������ݿɵó�������
��ѹ���䣬�����еĵ��������ĵ���ɷ���
��ѹ���䣬�����еĵ��������ĵ���ɷ���
�������о�����������ϵʱ����֤��ֵ�������˵�ѹ3V���䣬����5���Ķ�ֵ�������ʵ�飬�ٻ���10���Ķ�ֵ����ʱ��ijͬѧû�иı们����������Ƭ��λ�ã���ô�����Ͽ��غ�ѹ����ʾ����
����
����
3V������ڡ�����С�ڡ����ڡ�������ʱ��Ӧ����
��
����ҡ��������ڻ���Ƭ��ʹ��ѹ����ʾ��Ϊ3V����������1�����ݵ�·ͼȷ����Ԫ�������ӷ�ʽ��Ȼ����ݵ�·ͼ����ʵ���·ͼ��
��2�����ӵ�·ʱ����Ҫ�Ͽ����������Ƶ�ѹ�������Сʱ��Ϊ��֤��ȫ�����Ӧѡ��ϴ����̣��պϿ���ǰ����ƬҪ���������ֵ����
��3��������û��ʾ�������·��·����ѹ����ʾ���������ѹ�������ĵ�·��·��
��4�����������رպϺ��ѹ��ʾ��Ϊ�㣬�ҵ�������ʾ���������ݱ���·��˵����������ݲ����ˣ�
��5���ٸ���ͼ�������������͵�ѹ����ʾ��������ʱ��ע���������̺ͷֶ�ֵ��
�ڽ�����һ�����ݴ���R=
�������ֵ�������ֵ��
�۷�����һ�е����ݣ��ó������͵�ѹ�Ĺ�ϵ�����費�䣬�����͵�ѹ�����ȣ�
��6���ٷ��������е����ݣ��ó������͵���Ĺ�ϵ����ѹ���䣬�����͵���ɷ��ȣ�
�����մ�����·�д���ѹ��֪ʶ��������·�ĵ���Խ�ֵõĵ�ѹԽ����Ҫ��С����R1���˵ĵ�ѹ���������������ĵ��裮
��2�����ӵ�·ʱ����Ҫ�Ͽ����������Ƶ�ѹ�������Сʱ��Ϊ��֤��ȫ�����Ӧѡ��ϴ����̣��պϿ���ǰ����ƬҪ���������ֵ����
��3��������û��ʾ�������·��·����ѹ����ʾ���������ѹ�������ĵ�·��·��
��4�����������رպϺ��ѹ��ʾ��Ϊ�㣬�ҵ�������ʾ���������ݱ���·��˵����������ݲ����ˣ�
��5���ٸ���ͼ�������������͵�ѹ����ʾ��������ʱ��ע���������̺ͷֶ�ֵ��
�ڽ�����һ�����ݴ���R=
| U |
| I |
�۷�����һ�е����ݣ��ó������͵�ѹ�Ĺ�ϵ�����費�䣬�����͵�ѹ�����ȣ�
��6���ٷ��������е����ݣ��ó������͵���Ĺ�ϵ����ѹ���䣬�����͵���ɷ��ȣ�
�����մ�����·�д���ѹ��֪ʶ��������·�ĵ���Խ�ֵõĵ�ѹԽ����Ҫ��С����R1���˵ĵ�ѹ���������������ĵ��裮
����⣺��1�����ݵ�·ͼ����ʵ���·����ͼ��ʾ��

��2��Ϊ������·�������ӵ�·ͼʱ������Ӧ��ʼ���ǶϿ��ģ�
�պϿ���ǰ����Ƭ��Ӧ���ڱ����������·�е���ֵ���
��3����ѹ����ʾ������������û��ʾ������ɴ������ԭ������ǵ���R��·����R��·�����������ʾ������ѹ��ʾ��Ϊ�㣻
��4�����ػ�û�պϣ���ѹ��ʾ�����������رպϺ��ѹ����ʾ����СΪ�㣬������ʾ���������˵�����ؽ����ݶ�·����������ݲ����ˣ�
��5������ͼ��֪��������������Ϊ0��0.6A���ֶ�ֵΪ0.02A��ʾ��Ϊ0.3A��
��ѹ��������Ϊ0��15V���ֶ�ֵΪ0.5V��ʾ��Ϊ6V��
���ɱ���������֪��R1=R2=R3=
=
=20��
���ɱ���������֪�����費�䣬��ѹ����Ϊԭ���ļ���������Ҳ����Ϊԭ���ļ�������֪��ѹ���䣬�����еĵ������ѹ�����ȣ�
��6�����ɱ���֪����ѹ���䣬��������Ϊԭ���ļ������������СΪԭ���ļ���֮һ����֪��ѹ���䣬�����еĵ��������ĵ���ɷ��ȣ�
����5���Ķ�ֵ�������ʵ�飬�ٻ���10���Ķ�ֵ����ʱ�����ݴ���ѹ��֪ʶ����ѹ����ʾ�������3V����ʱӦ��������������ֵ������Ƭ���һ�����
�ʴ�Ϊ����1������ͼ����2���Ͽ������3����·����4���ѿ���S�����ڵ�������ˣ���5����
��20�����۵�ѹ���䣬�����еĵ��������ĵ���ɷ��ȣ���6���ٵ�ѹ���䣬�����еĵ��������ĵ���ɷ��ȣ��ڴ��ڣ��ң�

��2��Ϊ������·�������ӵ�·ͼʱ������Ӧ��ʼ���ǶϿ��ģ�
�պϿ���ǰ����Ƭ��Ӧ���ڱ����������·�е���ֵ���
��3����ѹ����ʾ������������û��ʾ������ɴ������ԭ������ǵ���R��·����R��·�����������ʾ������ѹ��ʾ��Ϊ�㣻
��4�����ػ�û�պϣ���ѹ��ʾ�����������رպϺ��ѹ����ʾ����СΪ�㣬������ʾ���������˵�����ؽ����ݶ�·����������ݲ����ˣ�
��5������ͼ��֪��������������Ϊ0��0.6A���ֶ�ֵΪ0.02A��ʾ��Ϊ0.3A��
��ѹ��������Ϊ0��15V���ֶ�ֵΪ0.5V��ʾ��Ϊ6V��
���ɱ���������֪��R1=R2=R3=
| U |
| I |
| 6V |
| 0.3A |
���ɱ���������֪�����費�䣬��ѹ����Ϊԭ���ļ���������Ҳ����Ϊԭ���ļ�������֪��ѹ���䣬�����еĵ������ѹ�����ȣ�
��6�����ɱ���֪����ѹ���䣬��������Ϊԭ���ļ������������СΪԭ���ļ���֮һ����֪��ѹ���䣬�����еĵ��������ĵ���ɷ��ȣ�
����5���Ķ�ֵ�������ʵ�飬�ٻ���10���Ķ�ֵ����ʱ�����ݴ���ѹ��֪ʶ����ѹ����ʾ�������3V����ʱӦ��������������ֵ������Ƭ���һ�����
�ʴ�Ϊ����1������ͼ����2���Ͽ������3����·����4���ѿ���S�����ڵ�������ˣ���5����
| ���� | 1 | 2 | 3 |
| ��ѹ��V�� | 2.0 | 4.0 | |
| ������A�� | 0.1 | 0.2 |
�����������о�����ĵ������ѹ������Ĺ�ϵ������Ӱ�����������������������Ҫ�ÿ��Ʊ���������̽����ͬʱ������ѧ���Դ���ѹ�����պ����⣮
�����˻�����������ʹ�ã�Ҫ�ж����С�ؼ��Ƿ�����Ƭ������һ��������֮��ľ����С��
�����˻�����������ʹ�ã�Ҫ�ж����С�ؼ��Ƿ�����Ƭ������һ��������֮��ľ����С��

��ϰ��ϵ�д�
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
�����Ŀ

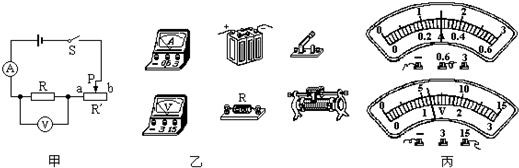
 С��Ϊ��̽����������ѹ������Ĺ�ϵ�����������ͼ��ʾ�ĵ�·ͼ������ʵ�����ʵ�飮
С��Ϊ��̽����������ѹ������Ĺ�ϵ�����������ͼ��ʾ�ĵ�·ͼ������ʵ�����ʵ�飮