��Ŀ����
Ϊ���о�����Ħ�����Ĵ�С����Щ�����йأ�С��ͬѧ����ʦ��ָ��������һϵ��ʵ�飮�����Dz���ʵ�鲽�裨ʵ������б��ֳ�ľ��ˮƽ�ҹ̶�����
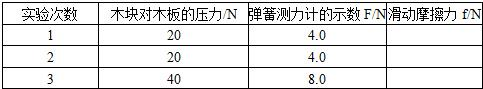
��һ�Σ���ľ��ƽ���ڳ�ľ���ϣ��õ��ɲ�����ˮƽ����ľ�飬ʹľ��������ֱ���˶�����ͼ����ʾ�����������ɲ����Ƶ�ʾ�����������±��У�
�ڶ��Σ���ľ�����ڳ�ľ���ϣ���ͬ���ķ��������飨��ͼ�ڣ���������Ӧ��ʾ����
�����Σ���������ͬľ�����һ��ƽ���ڳ�ľ���ϣ�����ͬ���ķ�����ľ�飨��ͼ�ۣ���������Ӧ��ʾ����
��1������֪ÿ��ľ�������Ϊ2kg���������С����ȫ�����ڵ����ݣ�
��2���Ƚ�
��3���ȽϢ١�������ʵ�����ݣ����Գ����ó��Ľ����ǣ�
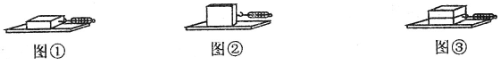
��һ�Σ���ľ��ƽ���ڳ�ľ���ϣ��õ��ɲ�����ˮƽ����ľ�飬ʹľ��������ֱ���˶�����ͼ����ʾ�����������ɲ����Ƶ�ʾ�����������±��У�
�ڶ��Σ���ľ�����ڳ�ľ���ϣ���ͬ���ķ��������飨��ͼ�ڣ���������Ӧ��ʾ����
�����Σ���������ͬľ�����һ��ƽ���ڳ�ľ���ϣ�����ͬ���ķ�����ľ�飨��ͼ�ۣ���������Ӧ��ʾ����
| ʵ����� | ľ���ľ���ѹ��/F | ���ɲ����Ƶ�ʾ�� | ����Ħ����/N |
| 1 | 4.0 | ||
| 2 | 4.0 | ||
| 3 | 8.0 |
��2���Ƚ�
1��2
1��2
����ʵ�����ݣ����Է��ֻ���Ħ�����Ĵ�С��Ӵ�����Ĵ�С�أ���3���ȽϢ١�������ʵ�����ݣ����Գ����ó��Ľ����ǣ�
�ڽӴ���ֲڳ̶���ͬʱ��ѹ��Խ����Ħ����Խ��
�ڽӴ���ֲڳ̶���ͬʱ��ѹ��Խ����Ħ����Խ��
��
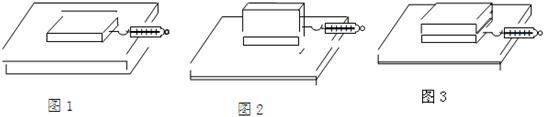
��������1������ƽ�������������Ħ����������ľ��������ľ���ľ���ѹ����
��2��̽��Ħ������С��Ӵ�������Ĺ�ϵ��Ӧ���ƽӴ���ֲڳ̶���ѹ����С��ȶ��Ӵ���������ͬ�������������ݣ�Ȼ����⣮
��3����������1��3��ʵ�����ݣ����ݿ��Ƶı�����ʵ������ó����ۣ�
��2��̽��Ħ������С��Ӵ�������Ĺ�ϵ��Ӧ���ƽӴ���ֲڳ̶���ѹ����С��ȶ��Ӵ���������ͬ�������������ݣ�Ȼ����⣮
��3����������1��3��ʵ�����ݣ����ݿ��Ƶı�����ʵ������ó����ۣ�
����⣺��1��ÿ��ľ�������Ϊ2kg��ÿ��ľ���ľ���ѹ��F=G=mg=2kg��10N/kg20N��
����ľ���ľ���ѹ��Ϊ40N����ƽ��������֪������Ħ���������������������±���ʾ��
��2����1��2����ʵ�����ݿ�֪�������Ӵ���Ĵֲڳ̶���������ѹ����ͬ���Ӵ���������ͬ�������Ļ���Ħ������ͬ���ɴ˿�֪������Ħ�����Ĵ�С��Ӵ�����أ�
��3���ɢ١�������ʵ�����ݿ�֪�������Ӵ���Ĵֲڳ̶���ͬʱ��������ѹ��Խ�������ܵ��Ļ���Ħ����Խ��
�ʴ�Ϊ����1�����ϱ���ʾ����2��1��2����3���ڽӴ���ֲڳ̶���ͬʱ��ѹ��Խ����Ħ����Խ��
����ľ���ľ���ѹ��Ϊ40N����ƽ��������֪������Ħ���������������������±���ʾ��
| ʵ����� | ľ���ľ���ѹ��/N | ���ɲ����Ƶ�ʾ��/N | ����Ħ����/N |
| 1 | 20 | 4.0 | 4.0 |
| 2 | 20 | 4.0 | 4.0 |
| 3 | 40 | 8.0 | 8.0 |
��3���ɢ١�������ʵ�����ݿ�֪�������Ӵ���Ĵֲڳ̶���ͬʱ��������ѹ��Խ�������ܵ��Ļ���Ħ����Խ��
�ʴ�Ϊ����1�����ϱ���ʾ����2��1��2����3���ڽӴ���ֲڳ̶���ͬʱ��ѹ��Խ����Ħ����Խ��
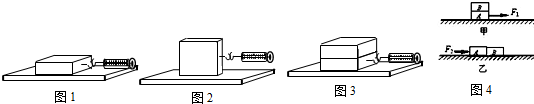
�������ڴ���ʵ���У���Ҫ̽������һ��������������̽�������е���������Ħ������С��ѹ����С���Ӵ�����Ĵ�С�Ĺ�ϵ�����У���ѹ����С�йأ���Ӵ�����Ĵ�С�أ���������Ӧ����֪�Ľ��ۣ�ͬʱ��ʵ���л��ֱ��õ���ת�����Ϳ��Ʊ�������Ҳ������Ӧ�����յ�����ѧ�Ļ����о�������

��ϰ��ϵ�д�
 ��ĩ100�ִ��غ�������ϵ�д�
��ĩ100�ִ��غ�������ϵ�д� Сѧ�������Ծ�ϵ�д�
Сѧ�������Ծ�ϵ�д�
�����Ŀ