��Ŀ����
����Ŀ����ͼ��ʾ��С��ѧϰ����������ɺ��������ٴξ���һ��ʵ��ϸ�ڡ�
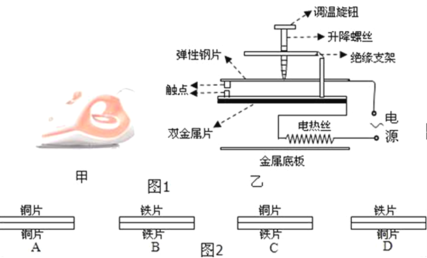
��1��С�����ü�ͼ��ʾ�ķ������������Ľ��࣬�����Ľ���Ϊ_____cm��
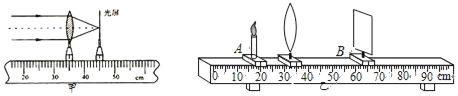
��2��ʵ��ǰ����װ������ʵ�����ģ�ʹ�������������λ������_____�ϣ�
��3��ʵ��ʱ��С����������ͼ��35cm�̶ȴ��������пɳ���_____��ѡ��Ŵ���С���ȴ����������������������������������ƶ�����������_____��ѡ��ܡ����ܡ����ٴ��ڹ����ϵõ��������
��4�����������Զ���������ı������λ�ã�������ڹ�����_____��ѡ����ҡ����࣬��_____��ѡ����ӡ����ϻ������ۿ������������ͬ��
���𰸡�10.0 ������ �Ŵ� �� �� ����
��������
��1����ͼ��֪���������Ľ����ǣ�![]() ��
��
��2����̽�����������ʱ��Ϊ��ʹ����ڹ��������룬��ȼ�������ʵ������ʱ����Ҫʹ�������������λ�������������ϣ�����������ġ����Ĺ��ġ����������Ĵ�����ͬһ�߶ȡ�
��3������������ͼ��35cm�̶ȴ�ʱ����ʱ����ǣ�![]() ����2f��u��f�������������֪������ʱ�ڹ����ϻ�õ��ɵ������Ŵ��ʵ�������������������������ƶ�������u��2fʱ���ɹ�·����֪�����ڹ��������ٴ��ڹ����ϵõ��������
����2f��u��f�������������֪������ʱ�ڹ����ϻ�õ��ɵ������Ŵ��ʵ�������������������������ƶ�������u��2fʱ���ɹ�·����֪�����ڹ��������ٴ��ڹ����ϵõ��������
��4����������Զ�����������������С�����ı������λ�ã��������ڹ�������࣬����������Ĥǰ��������������ۿ�����������ͬ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��ij����ʵ��С���ͬѧ����ͼ��ʾ�����IJ������С�2.5V��������С����L�ĵ繦�ʣ����е�Դ��ѹ��Ϊ6V�������������ϱ��С�50�� 1A��������
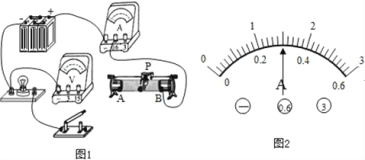
��1������ͼ���ñʻ��ߴ��浼�ߣ���ʵ��ͼ����������Ҫ���ѹ��������ѡȡ���������
��______��
��2��ʵ������У��������������ԶԵ�·�𱣻����ã���˱պϿ���ǰ����ƬӦ�Ƶ�_____�ˣ�ѡ�A����B������
��3��ʵ��С���ͬѧʵ�������������ݣ�
U/V | 0.5 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 |
I/A | 0.14 | 0.24 | 0.34 | 0.44 | 0.50 | 0.54 |
�����ݿ�֪������ѹU��_____Vʱ�������������⡣С���ݵĶ����ԼΪ_____W��
��ͬѧ�������ݺ��ֱ�����ͨ��С���ݵĵ���ֵ���ѹֵ�������ȣ��������������ϣ�����Ϊ���ܵ�ԭ����_____��
��4���ҵ�ԭ���ͬѧ����һ�������滻��С���ݡ�����̽���˵������ѹ�Ĺ�ϵ�����ڵó��ˡ�ͨ������ĵ���ֵ���ѹֵ�����ȡ������������Ǽ���̽���ˡ����������Ĺ�ϵ�������ǽ����˶��ʵ�飬ʵ���������±���ʾ�����еڶ���ʵ��ʱ��������ʾ����ͼ��ʾ���������СΪ_____A��
ʵ����� | U��V�� | R������ | I��A�� |
1 | 3 | 5 | 0.6 |
2 | 10 | ||
3 | 15 | 0.2 |
�����������ݿ�֪����_____һ��ʱ��ͨ������ĵ�������ε���ĵ����_____��
��5����ʵ���в����˶������ݣ���ʦ����ͬѧ�ǣ�������ʵ���ж���Ҫ���ж�β������е���Ϊ�˴Ӳ�ͬ������ҵ��ձ���ɣ��е���Ϊ�˶�β�����ƽ��ֵ��С������Ϊ����ʵ���ж�β�����Ŀ���뱾ʵ����ͬ����_____ ������ţ���
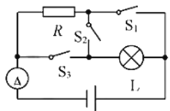
��̽��������·���˵ĵ�ѹ�����ĸ����ֵ�·�ĵ�ѹ֮��Ĺ�ϵ��
���÷�����������ֵ�������ֵ��
�۲�������ij��ȡ�