��Ŀ����
��2012?������ģ�⣩С��ͬѧ����ͼ��ʾ��װ�öԱ����ȣ�����ʵ���¼�ֱ�����˱��ۻ�ʱ��ˮ����ʱ�¶���ʱ��仯��ͼ����ͼ��������ʾ������ش�

��1��ͼ���У��¶ȼƵ�ʾ��Ϊ
��2����ͼ���У�
��3����ͼ����֪��ˮ�ķе���
��4����������С���ͼ��ʱָ����ͼ���е�GH�β��Ǹ���ʵ��IJ������ݻ������ģ�������ô˵��������

��1��ͼ���У��¶ȼƵ�ʾ��Ϊ
92
92
�森��2����ͼ���У�
BC
BC
�α�ʾ�����ۻ����̣�ѡ�AB������BC����CD�������ڵ�10���ӣ���������ʴ�����Һ���̬
��Һ���̬
��ѡ���̬������Һ̬����Һ���̬��������3����ͼ����֪��ˮ�ķе���
98
98
�棬��˵����ʱˮ���Ϸ�����ѹС��
��
��ѡ����ڡ�����С�ڡ����ڡ���һ��������ѹ����FG�Σ�ˮ����������
����
��ѡ��������ֲ��䡱��С��������4����������С���ͼ��ʱָ����ͼ���е�GH�β��Ǹ���ʵ��IJ������ݻ������ģ�������ô˵��������
ˮ����ʱ�¶ȱ��ֲ���
ˮ����ʱ�¶ȱ��ֲ���
����������1���¶ȼƵĶ���������ȷ�����ϻ������£�ȷ��ÿһ������ÿһ��С���������ʾ������2�������ۻ�ʱ�������������¶ȱ��ֲ��䣻�������ۻ������д��ڹ�Һ����״̬��
��3����Һ�����ʱ�����������¶ȱ��ֲ��䣬���������¶���Һ��ķе㣮
��һ������ѹ��ˮ�ķе���100�棬�е����ѹ�йأ���ѹԽ�ͣ��е�Խ�ͣ�
�۸ı����ܵ����ַ������ȴ��ݺ�������
��4��Һ�����ʱ�����������¶ȱ��ֲ��䣮��������ص���з�����
��3����Һ�����ʱ�����������¶ȱ��ֲ��䣬���������¶���Һ��ķе㣮
��һ������ѹ��ˮ�ķе���100�棬�е����ѹ�йأ���ѹԽ�ͣ��е�Խ�ͣ�
�۸ı����ܵ����ַ������ȴ��ݺ�������
��4��Һ�����ʱ�����������¶ȱ��ֲ��䣮��������ص���з�����
����⣺��1����̶����¶ȼƵ��·���Һ��Զ����̶ȣ������ϣ�ÿһ��������10�棬ÿһ��С�����1�棬ʾ����92�森
��2�����ͼ������BC�������������¶ȱ��ֲ��䣬����BC�α������ۻ����̣����ۻ�ʱ����Һ���棬�ǹ�Һ���̬��
��3�����綡ͼ��ˮ��FG�Σ����������������¶ȱ���98�治�䣬���Դ�ʱˮ�ķе���98�森
��һ������ѹ��ˮ�ķе���100�森����Ϊ��ѹԽ�ͣ��е�Խ�ͣ����Դ�ʱ����ѹ����һ������ѹ��
����FG�Σ�ˮ���ϴӾƾ�������������ˮ������������������
��4����Ϊˮ����ʱ�����������������¶ȱ��ֲ��䣬����������ߵ���������GH�β��Ǹ���ʵ��IJ������ݻ������ģ�
�ʴ�Ϊ����1��92����2��BC����Һ���̬����3��98��С�ڣ�����4��ˮ����ʱ�¶ȱ��ֲ��䣮
��2�����ͼ������BC�������������¶ȱ��ֲ��䣬����BC�α������ۻ����̣����ۻ�ʱ����Һ���棬�ǹ�Һ���̬��
��3�����綡ͼ��ˮ��FG�Σ����������������¶ȱ���98�治�䣬���Դ�ʱˮ�ķе���98�森
��һ������ѹ��ˮ�ķе���100�森����Ϊ��ѹԽ�ͣ��е�Խ�ͣ����Դ�ʱ����ѹ����һ������ѹ��
����FG�Σ�ˮ���ϴӾƾ�������������ˮ������������������
��4����Ϊˮ����ʱ�����������������¶ȱ��ֲ��䣬����������ߵ���������GH�β��Ǹ���ʵ��IJ������ݻ������ģ�
�ʴ�Ϊ����1��92����2��BC����Һ���̬����3��98��С�ڣ�����4��ˮ����ʱ�¶ȱ��ֲ��䣮
��������1���ܷ��������ۻ�ͼ���Һ����ڵ�ͼ����ȷ�������ʵ�״̬��
��2�����վ����ۻ��������ص㡢����Һ������������ص㣮
��2�����վ����ۻ��������ص㡢����Һ������������ص㣮

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
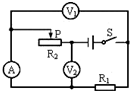 ��2012?������ģ�⣩��ͼ��ʾ��·�У���Դ��ѹ���ֲ��䣬�պϿ���S������������R2�Ļ�ƬP�����ƶ����ڴ˹����У�����A��ʾ��
��2012?������ģ�⣩��ͼ��ʾ��·�У���Դ��ѹ���ֲ��䣬�պϿ���S������������R2�Ļ�ƬP�����ƶ����ڴ˹����У�����A��ʾ��
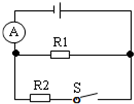 ��2012?������ģ�⣩��ͼ��ʾ����Դ��ѹ���䣬R1=30��������S�Ͽ�ʱ��������ʾ��0.4A��S�պ�ʱ��������ʾ��1.2A����
��2012?������ģ�⣩��ͼ��ʾ����Դ��ѹ���䣬R1=30��������S�Ͽ�ʱ��������ʾ��0.4A��S�պ�ʱ��������ʾ��1.2A����