��Ŀ����
����Ŀ��Ϊ����֤������·�ĵ����ص㣬Сޱ�������ͼ����ʾ�ĵ�·����ʵ�顣


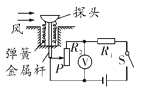
(1)�����ӵ�·ʱ������Ӧ����________״̬���պϿ��غ�����ָ����ͼ����ʾ��ԭ����____________________��
(2)��������Сޱ�Ƚ�����������L1���ڵ�֧·���պϿ��غ۲쵽��L2���⣬����L1�����⣬��������ʾ��Ϊ�㣬��·���ܴ��ڵĹ����ǣ�________________________��
(3)�ų����Ϻ��������L1��L2֧·��·�ϵĵ����ֱ�ΪI1��I2��I��������ʾ����ͼ������������ʾ���ɶ�����I1��0.5 A��I2��________A��I��________A�����ݲ�������������������Χ�ڣ�����Ϊ������·�и�·������֧·�����Ĺ�ϵ�ǣ�____________________��д����ϵʽ���ɣ���
(4)Ϊ����֤���۵��ձ��ԣ�Сޱ�����˸�����ͬ���ĵ��ݼ���ʵ��ķ���������֤���㻹���Բ��õķ����ǣ�________________________________��
���𰸡��Ͽ� �����������������ӷ��� L1�������������· 0.52 1.0 I��I1��I2 ���뻬�����������ڻ�Ƭλ�ã���ı��Դ��ѹ��
��������
(1)�����ӺͲ�ӵ�·ʱ��Ϊ�˱�֤��·Ԫ�����˵İ�ȫ��Ӧ�öϿ����أ���ͼ�ҵ�����ָ�뷴��ƫת��ԭ���ǵ����������������ӷ��ˣ�(2)���Ʋ���������еƶ�·�����ƶ����ᷢ�⣬��Ŀ��L2���⣬��L1�����⣬��ֻ���ǵ�L1����֧·��·����L1���������·��(3)����ͼ��������ʾ����֪��ͨ��L2�ĵ���I2��0.52 A��ͨ����·�ĵ�����I��1.0 A����Ϊ1.0 A��0.5 A��0.52 A����������Χ���У�I��I1��I2����������·�У���·�������ڸ�֧·����֮�ͣ�(4)Ϊ����֤���۵��ձ��ԣ����˲��ø�����ͬ���ĵ��ݼ���ʵ��ķ���������֤�⣬�����Բ��õķ����ǽ��뻬����������ͨ�����ڻ�Ƭλ�øı��·�еĵ�����ı��Դ��ѹ��

 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�