��Ŀ����
һ��ʵ����ϣ���ʦ�ṩ��ͬѧ���������ģ�һ���ѵ��õ���ƽ�������룩����ֻ��ȫ��ͬ���ձ���һֻ��Ͳ��ˮ���ιܣ�Ҫ����������������һ���Ͻ����ܶȡ�ijͬѧ��ƺ�ʵ�鷽���������²�����
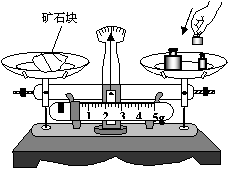
��1������ֻ���ձ��ֱ������ƽ�����������ڣ��ѺϽ����������ձ��ڡ�
��2�� ��ֱ����ƽƽ�⡣
��3�����ձ���ˮ�������Ͳ�У����ˮ���������ͼa��ʾ������ϸ��˨�úϽ�飬���������ͼa����Ͳ�У�����ˮ�ͺϽ������������ͼb������Ͻ����ܶ�Ϊ �K/m3
��4������������ĺϽ���ܶ�ֵ����ʵֵ���ƫС��������ԭ���ǣ� ��
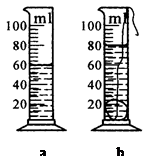
��1������ֻ���ձ��ֱ������ƽ�����������ڣ��ѺϽ����������ձ��ڡ�
��2�� ��ֱ����ƽƽ�⡣
��3�����ձ���ˮ�������Ͳ�У����ˮ���������ͼa��ʾ������ϸ��˨�úϽ�飬���������ͼa����Ͳ�У�����ˮ�ͺϽ������������ͼb������Ͻ����ܶ�Ϊ �K/m3
��4������������ĺϽ���ܶ�ֵ����ʵֵ���ƫС��������ԭ���ǣ� ��

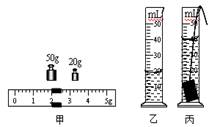
��2���������ձ��ڼ�ˮ�����õιܵ��� ��3��3��103 ��4���������ձ���ˮ������Ͳʱ��ˮ�������ڣ����ºϽ������ƫС��
�����������Ϊ��ͬѧ��Ƶ���ͼ��������ƽ�Ǹ��ȱ۸ܸˣ��������ձ���ˮ���������������ձ��ںϽ���������ȣ���������Ͳ����������ձ���ˮ�������ͨ��m=��V������Ͻ���������������ˮ������Ͻ��������ͨ����=m/V����Ͻ����ܶȡ����ԣ�����ˮ����������������飻�Ͻ�������m=��ˮV1=1g/cm3��60cm3=60g���Ͻ������V=V2-V1=(80-60)cm3=20cm3���Ͻ����ܶȦ�=m/V=60g/20cm3=3g/cm3=3��103�K/m3�����ڲ��裨3�����ձ���ˮ�������Ͳ��ʱ��ˮ���в��������²����ĺϽ�������ƫС������ĺϽ���ܶ�ֵ����ʵֵ���ƫС��

��ϰ��ϵ�д�
�����Ŀ


 ��
�� ʯ���ߣߣߣߣߣ���
ʯ���ߣߣߣߣߣ���