��Ŀ����
��֪������������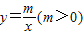 ��ͼ���ڵ�һ���ķ�֧����n����A1��1��y1����A2��2��y2��������An��n��yn������ֱ��A1A2�Ľ���ʽΪy=k1x+b1��A2A3�Ľ���ʽΪy=k2x+b2������AnAn+1�Ľ���ʽΪy=knx+bn��
��ͼ���ڵ�һ���ķ�֧����n����A1��1��y1����A2��2��y2��������An��n��yn������ֱ��A1A2�Ľ���ʽΪy=k1x+b1��A2A3�Ľ���ʽΪy=k2x+b2������AnAn+1�Ľ���ʽΪy=knx+bn����1����m=1ʱ��k1=______��
��2����m=1ʱ��k1+k2+k3=______��
��3���ٵ�m=2ʱ����k1+k2+k3+��+k20��ֵ����д�������̣�
����m��n��ʾk1+k2+k3+��+kn��ֵ��ֱ��д���������
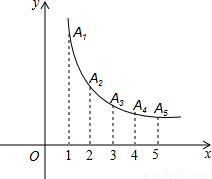
���𰸡���������1���ɷ����������Ľ���ʽy=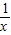 ��ȷ����A1������Ϊ��1��1������A2������Ϊ��2��
��ȷ����A1������Ϊ��1��1������A2������Ϊ��2�� �����ٰ����Ǵ���y=k1x+b1�õ�k1+b1=1�٣�2k1x+b1=
�����ٰ����Ǵ���y=k1x+b1�õ�k1+b1=1�٣�2k1x+b1= �ڣ�Ȼ���â�-�ٿ����k1=
�ڣ�Ȼ���â�-�ٿ����k1= -1=-
-1=- ��
��
��2����m=1ʱ�������������Ľ���ʽΪy=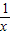 ����ȷ����A1������Ϊ��1��1������A2������Ϊ��2��
����ȷ����A1������Ϊ��1��1������A2������Ϊ��2�� ������A3������Ϊ��3��
������A3��������3�� ������A4��������4��
������A4������Ϊ��4�� �����루1��һ���õ�k2=
�����루1��һ���õ�k2= -
- ��k3=
��k3= -
- ���õ�k1+k2+k3��ֵ��
���õ�k1+k2+k3��ֵ��
��3���ٵ�m=2ʱ�������������Ľ���ʽΪy=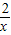 ����ȷ����A1����Ϊ��1��2������A2����Ϊ��2��
����ȷ����A1����Ϊ��1��2������A2����Ϊ��2�� ������A3������Ϊ��3��
������A3��������3�� ������A4��������4��
������A4��������4�� ����������A20������20��
����������A20������20��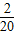 ������A21������21��
������A21����Ϊ��21��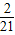 �������գ�1���õ�k1=
�������գ�1���õ�k1= -
- ��k2=
��k2= -
- ��k3=
��k3= -
- ������k20=
������k20=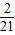 -
-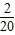 ����k1+k2+k3+��+k20=
����k1+k2+k3+��+k20= -
- +
+ -
- +
+ -
- +��+
+��+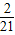 -
-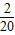 ��Ȼ����мӼ����㼴�ɣ�
��Ȼ����мӼ����㼴�ɣ�
���ȵõ���A1����Ϊ��1��m������A2����Ϊ��2��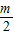 ������A3������Ϊ��3��
������A3��������3��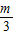 ������A4��������4��
������A4��������4��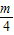 ����������An������n��
����������An������n��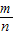 ������An+1������n+1��
������An+1����Ϊ��n+1��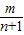 ������ͬ���ɵõ�k1=
������ͬ���ɵõ�k1=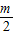 -m��k2=
-m��k2=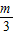 -
-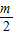 ��k3=
��k3=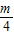 -
-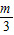 ������kn=
������kn=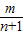 -
-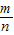 ����k1+k2+k3+��+kn=
����k1+k2+k3+��+kn=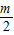 -m+
-m+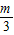 -
-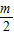 +
+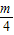 -
-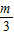 +��+
+��+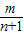 -
-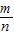 ��Ȼ����з�ʽ�ļӼ����㼴�ɣ�
��Ȼ����з�ʽ�ļӼ����㼴�ɣ�
����⣺��1����m=1ʱ�������������Ľ���ʽΪy=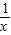 ��
��
���A1��������1��1������A2��������2�� ����
����
�ѵ�A1��1��1������A2��2�� ������y=k1x+b1��
������y=k1x+b1��
k1+b1=1�٣�
2k1x+b1= ��
��
���-�ٵ�k1= -1=-
-1=- ��
��
�ʴ�Ϊ- ��
��
��2����m=1ʱ�������������Ľ���ʽΪy=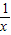 ��
��
��A1��������1��1������A2��������2�� ������A3��������3��
������A3��������3�� ������A4��������4��
������A4��������4�� ����
����
�루1��һ����k2= -
- ��k3=
��k3= -
- ��
��
��k1+k2+k3= -1+
-1+ -
- +
+ -
- =-1+
=-1+ =-
=- ��
��
�ʴ�Ϊ- ��
��
��3���ٵ�m=2ʱ�������������Ľ���ʽΪy=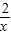 ��
��
���A1������1��2������A2������2�� ������A3��������3��
������A3��������3�� ������A4��������4��
������A4��������4�� ����������A20������20��
����������A20������20��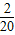 ������A21������21��
������A21������21��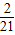 ����
����
�루1��һ����k1= -
- ��k2=
��k2= -
- ��k3=
��k3= -
- ������k20=
������k20=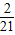 -
-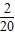 ��
��
��k1+k2+k3+��+k20= -
- +
+ -
- +
+ -
- +��+
+��+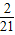 -
-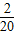 =-2+
=-2+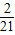 =-
=-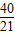 ��
��
�ڵ�A1����Ϊ��1��m������A2����Ϊ��2��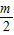 ������A3������Ϊ��3��
������A3��������3��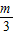 ������A4��������4��
������A4��������4��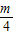 ����������An������n��
����������An������n��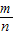 ������An+1������n+1��
������An+1������n+1��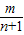 ����
����
�루1��һ����k1=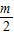 -m��k2=
-m��k2=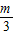 -
-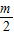 ��k3=
��k3=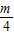 -
-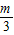 ������kn=
������kn=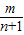 -
-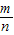 ��
��
��k1+k2+k3+��+kn=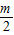 -m+
-m+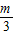 -
-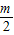 +
+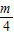 -
-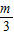 +��+
+��+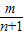 -
-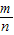 =-m+
=-m+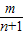 =-
=-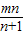 ��
��
���������⿼���˷����������ۺ��⣺���ڷ���������ͼ���ϣ������������������ʽ�����ô���ϵ���������Ľ���ʽ���������շ������ʽ�����㣮
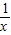 ��ȷ����A1������Ϊ��1��1������A2������Ϊ��2��
��ȷ����A1������Ϊ��1��1������A2������Ϊ��2�� �����ٰ����Ǵ���y=k1x+b1�õ�k1+b1=1�٣�2k1x+b1=
�����ٰ����Ǵ���y=k1x+b1�õ�k1+b1=1�٣�2k1x+b1= �ڣ�Ȼ���â�-�ٿ����k1=
�ڣ�Ȼ���â�-�ٿ����k1= -1=-
-1=- ��
����2����m=1ʱ�������������Ľ���ʽΪy=
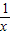 ����ȷ����A1������Ϊ��1��1������A2������Ϊ��2��
����ȷ����A1������Ϊ��1��1������A2������Ϊ��2�� ������A3������Ϊ��3��
������A3��������3�� ������A4��������4��
������A4������Ϊ��4�� �����루1��һ���õ�k2=
�����루1��һ���õ�k2= -
- ��k3=
��k3= -
- ���õ�k1+k2+k3��ֵ��
���õ�k1+k2+k3��ֵ����3���ٵ�m=2ʱ�������������Ľ���ʽΪy=
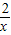 ����ȷ����A1����Ϊ��1��2������A2����Ϊ��2��
����ȷ����A1����Ϊ��1��2������A2����Ϊ��2�� ������A3������Ϊ��3��
������A3��������3�� ������A4��������4��
������A4��������4�� ����������A20������20��
����������A20������20��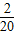 ������A21������21��
������A21����Ϊ��21��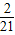 �������գ�1���õ�k1=
�������գ�1���õ�k1= -
- ��k2=
��k2= -
- ��k3=
��k3= -
- ������k20=
������k20=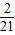 -
-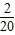 ����k1+k2+k3+��+k20=
����k1+k2+k3+��+k20= -
- +
+ -
- +
+ -
- +��+
+��+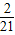 -
-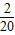 ��Ȼ����мӼ����㼴�ɣ�
��Ȼ����мӼ����㼴�ɣ����ȵõ���A1����Ϊ��1��m������A2����Ϊ��2��
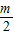 ������A3��������3��
������A3��������3��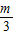 ������A4��������4��
������A4��������4��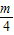 ����������An������n��
����������An������n��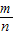 ������An+1������n+1��
������An+1����Ϊ��n+1��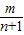 ������ͬ���ɵõ�k1=
������ͬ���ɵõ�k1=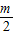 -m��k2=
-m��k2=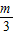 -
-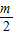 ��k3=
��k3=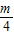 -
-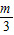 ������kn=
������kn=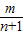 -
-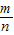 ����k1+k2+k3+��+kn=
����k1+k2+k3+��+kn=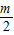 -m+
-m+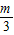 -
-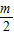 +
+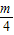 -
-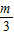 +��+
+��+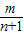 -
-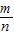 ��Ȼ����з�ʽ�ļӼ����㼴�ɣ�
��Ȼ����з�ʽ�ļӼ����㼴�ɣ�����⣺��1����m=1ʱ�������������Ľ���ʽΪy=
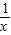 ��
�����A1��������1��1������A2��������2��
 ����
�����ѵ�A1��1��1������A2��2��
 ������y=k1x+b1��
������y=k1x+b1��k1+b1=1�٣�
2k1x+b1=
 ��
�����-�ٵ�k1=
 -1=-
-1=- ��
���ʴ�Ϊ-
 ��
����2����m=1ʱ�������������Ľ���ʽΪy=
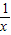 ��
����A1��������1��1������A2��������2��
 ������A3��������3��
������A3��������3�� ������A4��������4��
������A4��������4�� ����
�����루1��һ����k2=
 -
- ��k3=
��k3= -
- ��
����k1+k2+k3=
 -1+
-1+ -
- +
+ -
- =-1+
=-1+ =-
=- ��
���ʴ�Ϊ-
 ��
����3���ٵ�m=2ʱ�������������Ľ���ʽΪy=
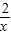 ��
�����A1������1��2������A2������2��
 ������A3��������3��
������A3��������3�� ������A4��������4��
������A4��������4�� ����������A20������20��
����������A20������20��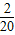 ������A21������21��
������A21������21��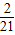 ����
�����루1��һ����k1=
 -
- ��k2=
��k2= -
- ��k3=
��k3= -
- ������k20=
������k20=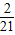 -
-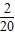 ��
����k1+k2+k3+��+k20=
 -
- +
+ -
- +
+ -
- +��+
+��+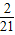 -
-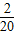 =-2+
=-2+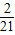 =-
=-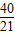 ��
���ڵ�A1����Ϊ��1��m������A2����Ϊ��2��
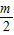 ������A3��������3��
������A3��������3��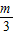 ������A4��������4��
������A4��������4��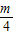 ����������An������n��
����������An������n��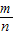 ������An+1������n+1��
������An+1������n+1��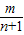 ����
�����루1��һ����k1=
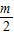 -m��k2=
-m��k2=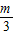 -
-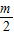 ��k3=
��k3=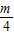 -
-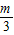 ������kn=
������kn=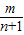 -
-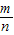 ��
����k1+k2+k3+��+kn=
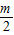 -m+
-m+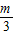 -
-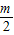 +
+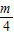 -
-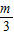 +��+
+��+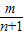 -
-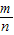 =-m+
=-m+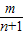 =-
=-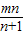 ��
�����������⿼���˷����������ۺ��⣺���ڷ���������ͼ���ϣ������������������ʽ�����ô���ϵ���������Ľ���ʽ���������շ������ʽ�����㣮

��ϰ��ϵ�д�
�����Ŀ
��֪һ��������������ͼ��Ҫȷ����������ı���ʽ��������Ҫ֪��ͼ���ϼ���������ꣿ�𣺣�������
| A��1�� | B��2�� | C��3�� | D��4�� |