��Ŀ����
����Ŀ����ϸ������̬ϵͳ�����۵���ۣ�����羮Ȼ�����̲�������İ��غ��ɣ����ж�ͼ����ʾ����̬ϵͳ�е�����˵���в���ȷ���ǣ� ��
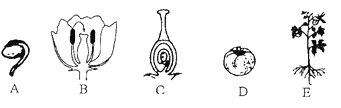
A. ͼ������ʾ���������ﶼ����ϸ������
B. �ۺ͢��ڽṹ����������Ǣ۶���ϵͳ�����Σ�������ϸ�����е��Ŵ����ʾ�����
C. ͼһ��ͼ���Т�ijϸ���ṹʾ��ͼ����ͼ�������������ϸ����ɶ���Ҷ����Ƚṹ
D. �ܵ�һ����ͷָ��ٴ����������������ʺ��������»ص�������������
���𰸡�D
��������
�⣺A����̬ϵͳ��������ɷֺͷ�����ɷ���ɵģ���������ɷְ�����̬ϵͳ�е�ȫ��������ݻ��Ӫ���������ķ�ʽ������ɷ��ֿ��Ի���Ϊ�����ߡ������ߺͷֽ��ߣ���������Ҫ��ָ��ɫֲ������߰������ֶ���ֽ�����Ҫ��ָϸ���������Ӫ������������ͼ�������������ߣ��������������ߣ����ֽ��ߣ������е������У�ֻ�в���û��ϸ���ṹ��ֲ����ϸ�������������ϸ�����ɵģ��ʲ��������⣮
B�����Ľṹ���Ϊ��ϸ������֯��������ϵͳ�������壻���Ľṹ���Ϊ��ϸ������֯��������ֲ���壮������Ϊ��ֲ�����ڵ��Ŵ����ʲ�ͬ�����µģ��ʲ��������⣮
C����ͼһ�о���Ҷ��������ṹ�����жϳ���ͼһ��ֲ��ϸ���Ľṹʾ��ͼ������ϸ���ڡ�ϸ��Ĥ��ϸ���ˡ�ϸ���ʡ�Һ�ݡ�Ҷ���壮����ϸ������ϸ��Ĥ��ϸ���ˡ�ϸ���ʵȽṹ��ϸ������ϸ���ڡ�ϸ��Ĥ��ϸ���ʡ�δ���ε�ϸ���ˣ������ϸ���ṹ����ϸ���ڡ�ϸ��Ĥ��ϸ���ʡ�ϸ���ˣ�����ֲ��ϸ������������Ľṹ����Ҷ����Ƚṹ�ʲ��������⣮
D�����Ƿֽ��ߣ����������ǽ���ֲ��IJ���Ⱥ��е��л���ֽ�ɼ�����黹���������У��ٽ������ʵ�ѭ��������̬ϵͳ�е����������ǵ��������ģ����������������»ص����������ڣ��ʷ������⣮
��ѡ��D

����Ŀ�����Ǻϳɼ�״�ټ��ص���Ҫԭ�ϣ�������������ȡһ�����ĵ⡣��ͼ����������ʵ����ա����ú��ų�����ʾ��ͼ����~�۷ֱ����ij���������̣�A��B��ʾij�����ʡ���ش��������⡣

![]()
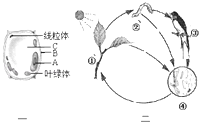
��1������ÿ������ĵ⣬Լ80%��Դ��ʳ�15%������ˮ����Щ��ͨ��ͼ��________������ţ����̣���Ҫ��________�����٣������ս���ѪҺ������Լ5%�ĵ����Կ�������Щ��ͨ��ͼ�е�________������ţ����̣�����________�ں�ëϸѪ�ܱ�ϸ������ѪҺ��
��2������________ϵͳ�ĵ⣬��ѪҺ����Ѹ�ٷֲ���ȫ��������һ���ֽ���________�У����ںϳɼ�״�ټ��ء�
��3�����ض�������������������Ҫ��________���á��磬��״�ټ����ܴٽ���л���ٽ�________�������ϵͳ���˷��ԡ���ͼ�е�ϸ��Ϊ��ϵͳ��ϸ����������________��ѡ�A����B�������Դ�����״�ټ��ء�
��4������ÿ���ų�һ�����ĵ⣬����Լ80%ͨ������ϵͳ�����ѪҺ�еĵ����Ⱦ�����С�����С���ڱڵ�________���ý���ԭ����������Һ�ų����⡣
��5��������������������٣���������״�ټ��ط��ڲ��㣬���¶��ּ�����Ϊ���о���Ũ�ȶԼ�״�ټ��ط�������Ӱ�죬�о����������ʵ�飬ʵ��������ͼ��ʾ��
��ͬ��Ũ���¼�״�ټ��ط������
��Ũ�� (nmol/L) | 1 | 2 | 4 | 7 | 9 | 10 | 30 | 50 | 75 | 100 | 120 | 140 |
��״�ټ��ط����� (ng/mL) | 9.58 | 16.22 | 24.50 | 26.80 | 37 | 32.5 | 26.5 | 26 | 24 | 23 | 15 | 9.75 |
����ͼ��֪����״�ټ��صķ��������ŵ�Ũ�ȵ�������________������һ��Ũ�ȣ���״�ټ��ط�������________��
�������о�����������ǵ���ʾ�ǣ���ÿ����ʳ�У�����Ӧ����________�⣬��ά��������������ļ�״�ټ���ˮƽ��