��Ŀ����
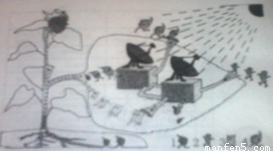
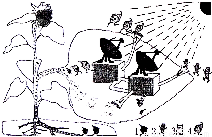
һ�����տ������۵ĸ����ʵ���������ԭ���ֵ�����������ӵ����������˼��ٱ�����ǧ������Щ���ӵĸ����ʣ�90%��95%��ֱ�ӻ������Թ������������л����ͼʾ��ɫֲ��ҶƬ���й�����õ�ʾ��ͼ��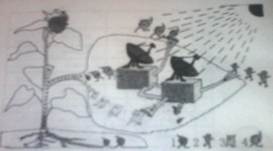
1.��������ͼʾ����ֲ�������ؽṹ����������ո�
��a���������Ҷ����һ�������л���Ĺ�������ô������ġ��������� ��
��ԭ�ϡ��� ������Ʒ���� ��
���������� ��
��b��[ 2 ]��[ 4 ]���������ġ��Ż����� ��[ 1 ]����ֲ������Ҫ��������� �����ա�[ 3 ]��ֲ�����ڵ�����ͨ���� ��
2.������Ȧ�У���ɫֲ��ͨ��������������л���������ǣ�
��
1.��a��Ҷ���� ������̼��ˮ �������л����ˮ�� ̫���ܣ�����ܣ�
��b������ ������ ɸ��
2.��ɫֲ��ͨ��������������л��ﲻ������������������������ֳ����Ҫ���һ�ֱ�ӻ��ӵ�Ϊ����Ȧ�е����������ṩ�˻�����ʳ����Դ��������Դ
�������������1��ֲ��Ĺ����������Ҷ���������ù��ܰѶ�����̼��ˮ�ϳ��л���ͷ�������ͬʱ�ѹ���ת��ɻ�ѧ�ܴ����ںϳɵ��л����еĹ��̣����ԭ���Ƕ�����̼��ˮ����Ʒ���л����������������Ҷ���壬�����ǹ��ܣ�����ϸ��֮���γɵ���״��С�׳�Ϊ���ף������ں������������õ������л�У���Ϊ������ˮ������ͨ·����ͨ�������ɱ���ϸ���Ŀ������������ڣ�ˮ����������Ҫ�Ķ��������������������µ��������ò������������������Ͼ�����Ҫ�����壬����Ϊֲ������ʧˮ�ġ��Ż�����Ҳ�����彻���ġ����ڡ���1��ˮ�֣��ǿ�ֲ�����ij����������������գ�3���л���л�����ֲ��������ͨ��ɸ�ܽ�������ġ�
2���ӹ�����õĸ�����Կ���������õ����壺���������ת�䣬��������ת����л��������������ֲ����ı�����ҲΪ���������Լ������ṩ��ʳ����Դ�����ͷ�����������������ã����������ת�䣬����һ�����У����ѹ���ת��Ϊ�������л����еĻ�ѧ�ܣ�����Ȼ���������Դ���۴Ӹ����ϸı��˵����ϵ��������ά�ִ����������Ͷ�����̼�����ƽ�⣬��ˣ����������һ����������ĸ������ϡ�
���㣺���⿼����ǹ�����õĸ��
����������Ϊ����֪ʶ�⣬�Ѷ�һ�㣬��������Ŀ�Ĺؼ�����������չ�����õĸ��

 ����������ϵ�д�
����������ϵ�д�һ�����տ������۵ĸ����ʵ���������ԭ���ֵ�����������ӵ����������˼��ٱ�����ǧ������Щ���ӵĸ����ʣ�90%��95%��ֱ�ӻ������Թ������������л����ͼʾ��ɫֲ��ҶƬ���й�����õ�ʾ��ͼ��
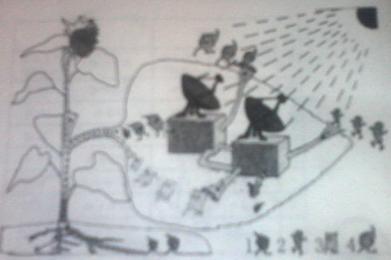 |
1.��������ͼʾ����ֲ�������ؽṹ����������ո�
��a���������Ҷ����һ�������л���Ĺ�������ô������ġ��������� ��
��ԭ�ϡ��� ������Ʒ���� ��
���������� ��
��b���� 2���ͣ� 4�����������ġ��Ż����� ���� 1������ֲ������Ҫ��������� �����ա��� 3����ֲ�����ڵ�����ͨ���� ��
2.������Ȧ�У���ɫֲ��ͨ��������������л���������ǣ�
![]()
��
 һ�����տ������۵ĸ����ʵ���������ԭ���ֵ�����������ӵ����������˼��ٱ�����ǧ������Щ���ӵĸ����ʣ�90%��95%��ֱ�ӻ������Թ������������л����ͼʾ��ɫֲ��ҶƬ���й�����õ�ʾ��ͼ��
һ�����տ������۵ĸ����ʵ���������ԭ���ֵ�����������ӵ����������˼��ٱ�����ǧ������Щ���ӵĸ����ʣ�90%��95%��ֱ�ӻ������Թ������������л����ͼʾ��ɫֲ��ҶƬ���й�����õ�ʾ��ͼ��