��Ŀ����
����Ŀ��ˮ������֮Դ��������ճ������빤ũҵ�������벻��ˮ��
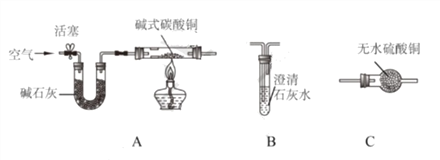
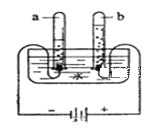
��1����ͼ1��ʾ��ͨ��һ��ʱ����Թ�1�����ռ�������Ϊ________����ʵ��˵��ˮ����________��ɵġ��÷�Ӧ�Ļ�ѧ����ʽ��________��
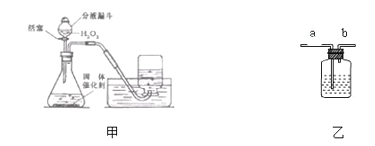
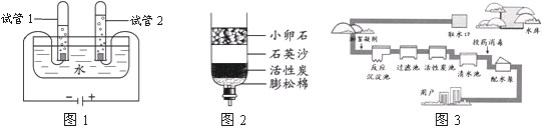
��2��ͼ2������������Ȼˮ�ļ���װ�ã���������������________������ţ���
A����ɱ������������������ B���ܵõ�����ˮ
C���ܰ�Ӳˮ��Ϊ��ˮ������ D����ʹ��Ȼˮ�����ɫ����
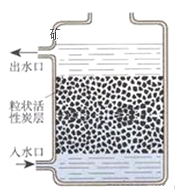
��3��ͼ3������ˮ���ľ�ˮ����ʾ��ͼ�����л���̿�ص�������________��ijͬѧ������������ˮ�Ƿ�ΪӲˮ������ʵ�鷽��Ϊ________��
��4��С�շ���һЩ��ѧʵ�鳣�������з�������ˮ�������ø�����ͬ���Իش�����ʵ��ָ��������ˮ�����á�



A����˿��������ȼ�ա���B���ⶨ�����������ĺ���������C����ˮ���ռ�����
A����ƿ�е�ˮ________��B��Ͳ�е�ˮ________��C����ƿ�е�ˮ________��
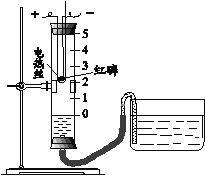
���𰸡�H2 ��Ԫ�غ���Ԫ�� 2H2O ![]() 2H2��+O2�� A��B��C �������� ȡ��ˮ�����������Թ��У��μ���������ˮ�����������϶ม������ԭˮ��ΪӲˮ����֮������Ӳˮ A����ƿ�е�ˮ��Ϊ�˷�ֹ�����ĸ������ʽ���ƿ�ף�ը�Ѽ���ƿ B��Ͳ�е�ˮ��Ϊ�˲ⶨ����ƿ�к���ȼ�������ĵ�������� C����ƿ�е�ˮ��Ϊ���ž�����ƿ�еĿ�����ͬʱ���ռ���������й۲����ռ���������
2H2��+O2�� A��B��C �������� ȡ��ˮ�����������Թ��У��μ���������ˮ�����������϶ม������ԭˮ��ΪӲˮ����֮������Ӳˮ A����ƿ�е�ˮ��Ϊ�˷�ֹ�����ĸ������ʽ���ƿ�ף�ը�Ѽ���ƿ B��Ͳ�е�ˮ��Ϊ�˲ⶨ����ƿ�к���ȼ�������ĵ�������� C����ƿ�е�ˮ��Ϊ���ž�����ƿ�еĿ�����ͬʱ���ռ���������й۲����ռ���������
��������
��1�����ˮ����������������������������Ϊ�������������Ϊ��1��2��ͬʱ˵��ˮ������������Ԫ����ɵģ����Ծݴ˽��
��2������ͼ2������������Ȼˮ�ļ���װ��ֻ�ܹ��˵�ˮ�еIJ��������ʽ��н��
��3�����ݻ���̿�����������Լ�������������ˮ�Ƿ�ΪӲˮ�÷���ˮ���н��
��4��������˿��������ȼ�յ�ע������ⶨ�����������ĺ�������ˮ�ռ�������ˮ�����ý��н����
��1�����ˮʱ����������������������������Ϊ������1�Թܺ͵�صĸ�������������1�Թܲ���������Ϊ������H2��������������Ԫ����ɣ�����������Ԫ����ɣ��ڻ�ѧ�仯��Ԫ�ص�����䣬˵��ˮ�����⡢������Ԫ����ɵģ��÷�Ӧ�Ļ�ѧ����ʽ��2H2O ![]() 2H2��+O2����
2H2��+O2����
��2��A����ˮ����������Ҫ���˺�ˮ�еIJ����Թ��壬����̿����ɫ�أ�����������ɱ�������ã���A����B����ˮ��������ֻ�ܳ�ȥ�����Թ������ʺ�ɫ�أ����ܳ�ȥ���������ʣ���B����C����ˮ�������ʲ��ܽ���ˮ�иơ�þ���Ӻ��������ܰ�Ӳˮ��Ϊ��ˮ����C����D������̿���������ԣ���ˮ���л���̿��Ҫ��������ˮ������ζ�����ʺ�ɫ�أ����˵�ˮ�еIJ��������ʣ�ʹ��Ȼˮ�����ɫ���壬��D��ȷ����ѡABC��
��3������̿���������ԣ��ܳ�ȥˮ��ɫ�غ���ζ�����ʣ�������������ˮ�Ƿ�ΪӲˮ�÷���ˮ��ʵ�鷽��Ϊ��ȡ��ˮ�����������Թ��У��μ���������ˮ�����������϶ม������ԭˮ��ΪӲˮ����֮������Ӳˮ��
��4����˿��������ȼ�ղ����������ȣ�ʵ���м���ƿ�е�ˮ��Ϊ�˷�ֹ�����ĸ������ʽ���ƿ�ף�ը�Ѽ���ƿ���ⶨ�����������ĺ���������ȼ�������˼���ƿ�е�������ƿ�ڵ���ѹ�½����ձ��е�ˮ����������ƿ�У������ˮ������������ĵ������������Ͳ�е�ˮ��Ϊ�˲ⶨ����ƿ�к���ȼ�������ĵ������������ˮ���ռ�����ʵ����Ӧ�������е�ѹǿԭ������ˮ�ȰѼ���ƿ��Ϊ�������û������������ѹǿΪ�������ռ���������뼯��ƿ��,ƿ��ѹǿ��������ˮѹ��ֱ������ƿ�е�ˮȫ���ų�����ˮ�ռ�������ˮ�����ã���Ϊ���ž�����ƿ�еĿ�����ͬʱ���ռ���������й۲����ռ�����������

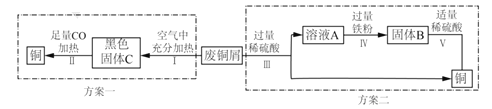
����Ŀ�����A��B��������ѡһ����������������𣬰�A�Ʒ֡�
A | B |
|
|
�����ǶȽ��ͣ� (1)Ʒ����ˮ����ɢ��ԭ����______�� (2)Ʒ������ˮ����ɢ�ٶȿ죬��ԭ����______�� | (1)ʵ��������______�� (2)ʵ�������______�� |
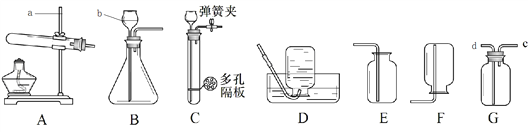
����Ŀ��������ͼװ�ý��п��������������ⶨʵ�顣
ʵ��װ�� | ʵ����� |
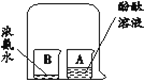
| I�������ܺ�ˮ���ڼ�������ˮ����ȼ�ճ�����������ף��������ӣ������ƶ�ˮ��������ˮ����ƽ�ڲ����ܵ���̶�λ�á� II����ͨ��Դ��������ȼ�գ��Ͽ���Դ�� III�����¶Ȼָ������£�ˮ���ٽ��벣����ʱ�������ƶ�ˮ��������ˮ����ƽ�� |
��1��ʵ���м�����������Ŀ����_________��
��2��ʵ������۲쵽��������_________��
��3��ʵ�����I��III�У������ƶ�ˮ��������ˮ����ƽ��Ŀ����________��