��Ŀ����
ijѧϰС��ͬѧ�þ��õĹ���������Һ����ͼ����ȡ������ȡ2g������������ƿ�У�Ȼ����������34g����������Һ����ַ�Ӧ��������з��������㣺

��С�����ݹ�����������Ԫ�ص������������������������������ʽΪ��
����������������34g��10%����100%
��34g��10%�� ��100%��3.2g��
��100%��3.2g��
��ʦָ��С���ļ����Ǵ���ģ�����Ϊ�����ԭ���� �� (����ĸ���)��
A�������������Ԫ�ص�����������������
B�����������е���Ԫ��û��ȫ��ת�Ƶ�������
��������ݻ�ѧ����ʽ����С�����������Ͽ���ȡ������������
��С��˼�����������ⲻ�ȶ�����Ȼ�ֽ⣬������Һ�����ʵ�����������С��ʵ������Ƶ���ƿ��ʣ�����ʵ���������35.2g���������ƿ����������Һ��ʵ�����ʵ�����������

��С�����ݹ�����������Ԫ�ص������������������������������ʽΪ��
����������������34g��10%����100%
��34g��10%��
 ��100%��3.2g��
��100%��3.2g����ʦָ��С���ļ����Ǵ���ģ�����Ϊ�����ԭ���� �� (����ĸ���)��
A�������������Ԫ�ص�����������������
B�����������е���Ԫ��û��ȫ��ת�Ƶ�������
��������ݻ�ѧ����ʽ����С�����������Ͽ���ȡ������������
��С��˼�����������ⲻ�ȶ�����Ȼ�ֽ⣬������Һ�����ʵ�����������С��ʵ������Ƶ���ƿ��ʣ�����ʵ���������35.2g���������ƿ����������Һ��ʵ�����ʵ�����������
(1)B (2)1.6g (3)O2=34g+2g��35.2g=0.8g H2O2%=5%
��������1�����ݹ������������������������ ��Ӧԭ�����з�����
��2�����ݹ�������ֽⷽ��ʽ�еĵ�����ϵ���з������
��3������������������������������������������������
�⣺��1����������ֽ���ȡ����ʱ��������ˮ�����������������е���Ԫ��û��ȫ��ת��Ϊ��������һ������ˮ�У��ʴ�Ϊ��B��
��2���⣺��������������Ϊx��
2H2O2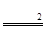 H2O+O2��
H2O+O2��
68 32
34��10% x
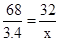
x=1.6g��
�������Ͽ���ȡ����������Ϊ��1.6g��
��3�����������غ㶨�ɿ�֪��������������Ϊ��O2=34g+2g-35.2g=0.8g
����Ҫ��������Ϊy��
2H2O2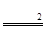 2H2O+O2��
2H2O+O2��
68 32
y 0.8g
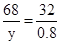
y=1.7g��
���� H2O2%=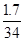 ��100%=5%��
��100%=5%��
�𣺹���������Һ��ʵ�����ʵ���������Ϊ5%��
��2�����ݹ�������ֽⷽ��ʽ�еĵ�����ϵ���з������
��3������������������������������������������������
�⣺��1����������ֽ���ȡ����ʱ��������ˮ�����������������е���Ԫ��û��ȫ��ת��Ϊ��������һ������ˮ�У��ʴ�Ϊ��B��
��2���⣺��������������Ϊx��
2H2O2
 H2O+O2��
H2O+O2��68 32
34��10% x
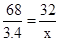
x=1.6g��
�������Ͽ���ȡ����������Ϊ��1.6g��
��3�����������غ㶨�ɿ�֪��������������Ϊ��O2=34g+2g-35.2g=0.8g
����Ҫ��������Ϊy��
2H2O2
 2H2O+O2��
2H2O+O2��68 32
y 0.8g
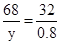
y=1.7g��
���� H2O2%=
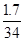 ��100%=5%��
��100%=5%���𣺹���������Һ��ʵ�����ʵ���������Ϊ5%��

��ϰ��ϵ�д�
�����Ŀ


