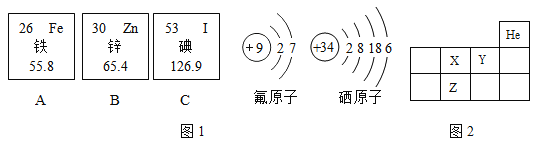
��Ŀ����
����Ŀ������ H2SO4�� CuSO4�Ļ����Һ��Ϊ�˷��������Һ�� H2SO4�� CuSO4�������������������ͼʵ�鷽������ע������ͭ��Һ�������ԣ�
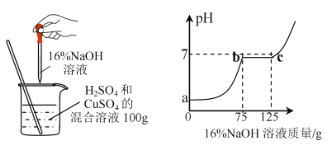
��1��c ������Һ�� NaOH ǡ�÷�Ӧ��ȫ���˵���Һ�е�����Ϊ_______��д��ѧʽ����
��2��������ͭ��Һ��Ӧ������������Һ������Ϊ__________g�������û����Һ�� CuSO4����������������д��������̣���___��
��3������ 100g �����Һ�в��ϼ��������������Ƶ���Һ�����㻭����������������Һ����������������������Ĺ�ϵͼ�����ڴ��������ͼ����ͼ��____��
��4����ʵ�鷽����֤��������������Ʒ������кͷ�Ӧ��ԭ����_______________��
���𰸡�Na2SO4 50 16% 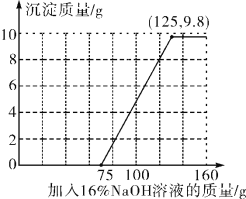 һ��ʼ������������Һ��������������������ʱ��˵���кͷ�Ӧ�Ѿ����
һ��ʼ������������Һ��������������������ʱ��˵���кͷ�Ӧ�Ѿ����
��������
��1����c�㣬�����Һ��NaOHǡ�÷�Ӧ��ȫ���˵���Һ�е�������Na2SO4��
��2����ͼ����pH�ı仯����֪����������ͭ��Ӧ���������Ƶ�����Ϊ��125g-75g=50g��
�⣺��û����Һ�� CuSO4��������������Ϊx��������ͭ������Ϊy��
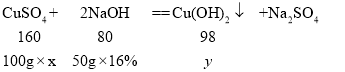
![]()
![]() ��
��![]()
�𣺸û����Һ��CuSO4��������������Ϊ16%�����16%
��3������100 g�����Һ�в��ϼ�����������������Һ����ͼ��֪��һ��ʼ�����������������ᷴӦ�����������Ƽ��뵽75gʱ���������ƿ�ʼ������ͭ��Ӧ���г������������������������ƣ��������ӣ����������Ƽ��뵽125gʱ������ͭ�Ѿ���Ӧ��ϣ�����������ͭ�������ɣ�2����Ϊ9.8g������ȫ��Ӧ�������Ϊ����125��9.8�����ټ��������������ƣ������������ӣ���������������Һ����������������������Ĺ�ϵ��ͼ��ʾ��
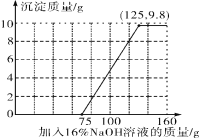
��4����ͼ��֪����ʵ�鷽��֤��������������Ʒ������кͷ�Ӧ�������ǣ�һ��ʼ������������Һ��������������������ʱ��˵���кͷ�Ӧ�Ѿ���ɣ����һ��ʼ������������Һ��������������������ʱ��˵���кͷ�Ӧ�Ѿ����

����Ŀ������ʵ����Ʋ��ܴﵽĿ�ĵ��ǣ� ��
ѡ�� | A | B | C | D |
ʵ����� |
|
|
|
|
ʵ�� Ŀ�� | ֤��������̼������ˮ | ֤��������̼�ܶȴ��ڿ��� | ֤��ͨ������� �Ƕ�����̼ | ֤��С�մ�����ϡ���ᷴӦ�ų� CO2 |
A. AB. BC. CD. D
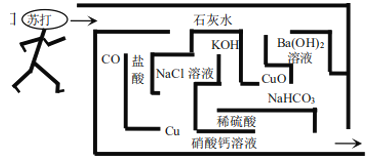
����Ŀ��Ϊ̽��CO2��NaOH��Һ�����ķ�Ӧ��ij��ȤС�鳢���ò�ͬ�ķ�ʽ����ʵ����
������������
��.20��ʱ������������ˮ�е��ܽ�ȼ��±���
���� | Na2CO3 | NaHCO3 | Ca(OH)2 | Ba(OH)2 |
�ܽ��/g | 21.5 | 9.6 | 0.165 | 3.89 |
��.��ʵ����������Na2CO3��Һ��NaHCO3��Һ��pH�ֱ�ԼΪ11.0��8.5��
��ʵ��̽����
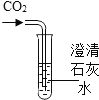
(1)ʵ��һ��С��ȡһ����CO2�Ŀ�Ȫˮƿ������һ������ˮ������š��ƿ������������ƿ�ӱ����С����ȡһ��ͬ�ij���CO2�Ŀ�Ȫˮƿ�������м�����ˮ�������NaOH��Һ������š��ƿ���������õ���ҺX����ʱ�۲쵽��������_________________________________��ʵ������С�������ֻ��Ȫˮƿ���Ա�ʵ���Ŀ����_________________________________��
(2)ʵ�����Ϊ����CO2��NaOH��Һ��Ӧ�IJ�����С��ȡʵ��һ������ҺX�����������еμ�BaCl2��Һ���а�ɫ�����������÷�Ӧ�Ļ�ѧ����ʽΪ_____________________________��ʵ���в��˽�BaCl2��Һ����CaCl2��Һ��ԭ����_____________________________________��
(3)ʵ������С��ȡʵ��һ������ҺX�����������м��������BaCl2��Һ������������ȡ�ϲ���Һ������1�η�̪��Һ��������Һ��____ɫ��֤����ҺX����NaOHʣ����ʵ������С��û��ֱ����������ҺX�е����̪��Һ��������___________________________________��
(4)ʵ��������ȤС�齫CO2����ͨ��һ��Ũ��һ������NaOH��Һ���������ֻ�ʵ�鼼���ⶨ��Ӧ��������Һ��pH���¶ȱ仯�������ͼ1��ͼ2��ʾ��
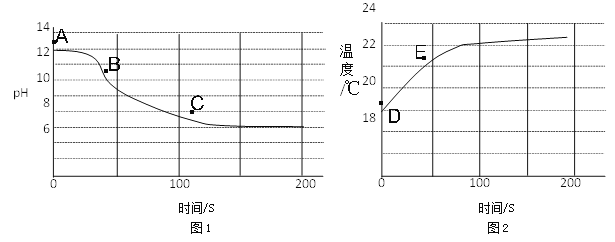
ͼ1����BC�η�����Ӧ�Ļ�ѧ����ʽΪ____________________________________��
ͼ2����DE���¶ȱ仯��ԭ����__________________________________________��
����˼������
(5)ʵ���CO2���١�NaOH������Na2CO3���ɵ����ʵı仯���Լ�___________ת�����ӽǶ�ά��̽��CO2��NaOH�����˷�Ӧ�������������ԵĻ�ѧ��Ӧ������ͨ���ִ������ֶν������ݲⶨ��ʵ�ַ�Ӧ���̵������ӻ�����