��Ŀ����
ij����ͭ��CuO����ĩ�л�������������ͭ��Cu2O��������һ����С��������ͼ��ʾװ�òⶨ����Cu2O�ĺ����������ͼʾ���ݻش��������⣺
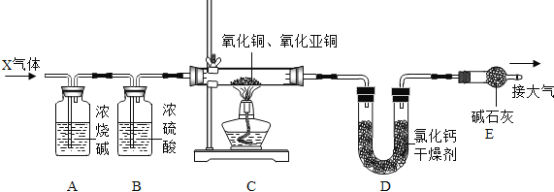
��1��X�dz�����ԭ������CO��H2�е�һ�֣����ܻ�������ˮ�Ͷ�����̼������������װ���ж�X�Ļ�ѧʽΪ______��Aװ�õ�������__________��
��2��Bװ�õ�������__________����ȱ��������ֱ�ӵ��·�Ӧ��_______װ���ڵ����ʵ�����ƫ�
��3��Eװ�õ�������_________�� ���ȱ��Eװ�ã���ⶨCu2O�ĺ�����ƫ_______�����С����
��4����֪��Ӧǰ������������Ϊ15.2g����ȫ��Ӧ��U�������ʵ�����������2.7����ʧ���Բ��ƣ�����ԭ�������Cu2O������Ϊ__________��
Cu��Zn�ĺϽ��Ϊ��ͭ���������ĵ����Ժ���ʴ�ԣ��������������������ij��ѧ��ȤС���ͬѧΪ�˲ⶨij��ͭ����ɣ�ȡ10g�û�ͭ��Ʒ���ձ��У������з�5�μ�����ͬ��������������ϡ���ᣬʹ֮��ַ�Ӧ��ÿ������ϡ�����������ʣ������������¼���±���
����ϡ�����������g�� | ��ַ�Ӧ��ʣ������������g�� | |
��1�� | 10 | m |
��2�� | 10 | 7.4 |
��3�� | 10 | 6.1 |
��4�� | 10 | 5.6 |
��5�� | 10 | n |
�Իش��������⣺
��1�����������ݿ�֪�����ʣ���5.6g����ijɷ���_____�������У�m=_____,n=_____��
��2����ͭ��Ʒ��п����������Ϊ_____��
��3����������ϡ�������������������Ϊ_____��
�±���Ca(OH)2��NaOH���ܽ�����ݡ���ش��������⣺
�¶�/�� | 0 | 20 | 40 | 60 | 80 | 100 | |
�ܽ��/g | Ca(OH)2 | 0.19 | 0.17 | 0.14 | 0.12 | 0.09 | 0.08 |
NaOH | 31 | 91 | 111 | 129 | 313 | 336 |
��1�������ϱ����ݣ�����Ca(OH)2��NaOH���ܽ�����ߣ���ͼ���ܱ�ʾNaOH�ܽ�����ߵ���_____����A��B����

��2��Ҫ���һƿ�ӽ����͵�Ca(OH)2��Һ��ɱ�����Һ�������ʩ�У�
�ټ����������ƣ��������¶ȣ��۽����¶ȣ��ܼ���ˮ��������ˮ���ٻָ���ԭ�¶ȣ�������ʯ�ҡ����д�ʩ��ȷ����_____��
A �ڢܢ� B �ۢ� C �٢ۢݢ� D �٢ڢݢ�
��3��20��ʱ��191g����NaOH��Һ������10gˮ���ٽ��µ�20�棬������NaOH���������Ϊ_____��
��4������20��ʱCa(OH)2�ı�����Һ������Һ���������м���һ����CaO��õ�����Һ������Һ������ʱ��Һ�����ʵ�����������_____�ף����������������������
��5������60��ʱ��Ca(OH)2��NaOH�������ʵı�����Һ����Ҫ�õ��ϴ�����NaOH���壬Ӧ��ȡ������������_____��