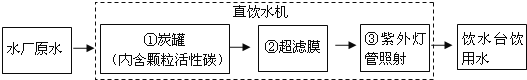
��Ŀ����
����Ŀ��С�մ�(��Ҫ�ɷ�ΪNaHCO3)�г����������Ȼ��ơ���ѧ��ȤС���ͬѧΪ�˲ⶨijƷ��С�մ���NaHCO3����������������������ʵ�飺������Ʒ�����ձ��У������������μ�ϡ���ᣬ�����ٲ�������Ϊֹ����õ��й��������±���ʾ��
���� | ��Ʒ | ���ĵ�ϡ���� | ��Ӧ�����Һ |
����(g) | 9 | 75.4 | 80 |
�Լ���(����������һλС��)��
(1)��Ʒ�е�NaHCO3������������
(2)������Һ��NaCl������������
���𰸡���1��93.3% ��2��8.1%
���������÷�Ӧ�Ļ�ѧ����ʽΪNaHCO3+HCl=NaCl+H2O+CO2��,��Ӧ�����ɵĶ�����̼���������Ϊ9 g+75.4 g-80 g=4.4 g,��Ʒ�е�NaHCO3��������������Һ��NaCl������������CO2�����������
�⣺��Ӧ�����ɵĶ�����̼���������Ϊ9 g+75.4 g-80 g=4.4 g��
����Ʒ�е�NaHCO3����Ϊx�����ɵ�NaCl������Ϊy
NaHCO3+HCl=NaCl+H2O+CO2��
84 58.5 44
x y 4.4 g
![]() x=8.4 g
x=8.4 g
![]() y=5.85 g
y=5.85 g
(1)��Ʒ�е�NaHCO3����������Ϊ��![]() ��100%��93.3%
��100%��93.3%
(2)��Ʒ��NaCl������=9 g -8.4 g=0.6 g����Һ��NaCl��������=0.6 g+5.85 g=6.45 g��������Һ��NaCl����������Ϊ��![]() ��100%��8.1%
��100%��8.1%

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
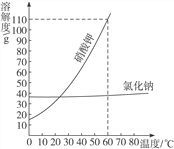
Сѧ��10����Ӧ����ϵ�д�����Ŀ���±���NaCl��KNO3�ڲ�ͬ����ʱ���ܽ�ȣ��ش�������
�¶�/�� | 10 | 20 | 30 | 40 | 50 | 60 | |
�ܽ��/g | NaCl | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 |
KNO3 | 20.9 | 31.6 | 45.8 | 63.9 | 85.5 | 110.0 | |
��1���������������ܽ�����¶�Ӱ��仯�ϴ����_________��
��2��60��ʱ����ͼʾ������
![]()
A��������_____(����������������������)��Һ��C����Һ����������______g��
��3��50��ʱ�����������ʵı�����Һ��100g���ֱ��������10gˮ���ٻָ���50����ʣ����Һ��������NaCl��Һ_____������������������������С������ KNO3��Һ��