��Ŀ����
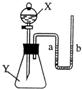 25����ͼ��ʾ����ƿ��ʢ������Y����Һ©����ʢ��Һ��X��U����ʢ�к�īˮ�����ڹ۲죩����ʼʱa��b����ͬһˮƽ�ߣ�����X������ƿ��ʱ������ش��������⣺
25����ͼ��ʾ����ƿ��ʢ������Y����Һ©����ʢ��Һ��X��U����ʢ�к�īˮ�����ڹ۲죩����ʼʱa��b����ͬһˮƽ�ߣ�����X������ƿ��ʱ������ش��������⣺��1����XΪϡ���ᣬYΪ��ʯ�ң���U����aҺ��
����
������ڡ��������ڡ����ڡ�����ͬ��bҺ�棻��2����XΪˮ��YΪ����粒��壬��U����aҺ��
����
bҺ�棻��3����XΪ����������Һ��YΪ������̼���壬��U�ι���aҺ��
����
bҺ�棬��Ӧ�Ļ�ѧ����ʽ��
2NaOH+CO2�TNa2CO3+H2O
����������1��ϡ����������ƻ��ʱ�ܹ��ų��������ȣ���ʹƿ��ѹǿ�����仯��
��2�����������ˮʱ���մ������ȣ���ʹƿ��ѹǿ�����䣻
��3��������̼�ܹ�������������Һ���գ���ʹƿ��ѹǿ�����䣻
��4�����ݷ�Ӧ��������P�������غ㶨�ɿ�����ȷ����д��ѧ����ʽ��
��2�����������ˮʱ���մ������ȣ���ʹƿ��ѹǿ�����䣻
��3��������̼�ܹ�������������Һ���գ���ʹƿ��ѹǿ�����䣻
��4�����ݷ�Ӧ��������P�������غ㶨�ɿ�����ȷ����д��ѧ����ʽ��
����⣺��1��ϡ����������ƻ��ʱ�ܹ��ų��������ȣ���ƿ�ڵĿ����������ͣ�ѹǿ���ߣ�������ڣ�
��2�����������ˮʱ���մ������ȣ���ƿ�ڵĿ����¶Ƚ��ͣ�ѹǿ��С��������ڣ�
��3������������Һ�ܹ����ն�����̼���壬�Ӷ�ʹ����ƿ�ڵ�ѹǿ��С��������ڣ�
�������ƺͶ�����̼��Ӧ�Ļ�ѧ����ʽΪ��2NaOH+CO2�TNa2CO3+H2O��
��2�����������ˮʱ���մ������ȣ���ƿ�ڵĿ����¶Ƚ��ͣ�ѹǿ��С��������ڣ�
��3������������Һ�ܹ����ն�����̼���壬�Ӷ�ʹ����ƿ�ڵ�ѹǿ��С��������ڣ�
�������ƺͶ�����̼��Ӧ�Ļ�ѧ����ʽΪ��2NaOH+CO2�TNa2CO3+H2O��
�����������Ҫ���ջ�ѧ����ʽ����д�����������ʵ����ʵȷ�������ݣ�ֻ���������ܶ���ط��������������ȷ���жϣ�

��ϰ��ϵ�д�
�����Ŀ
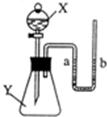
 9����ͼ��ʾ����ƿ��ʢ������X���ι���ʢ��Һ��Y������ѹ�ιܽ�ͷ��ʹҺ��Y����ƿ�У���һ����ɼ�С����a��������X��Һ��Y�������ǣ�������
9����ͼ��ʾ����ƿ��ʢ������X���ι���ʢ��Һ��Y������ѹ�ιܽ�ͷ��ʹҺ��Y����ƿ�У���һ����ɼ�С����a��������X��Һ��Y�������ǣ�������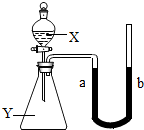 ��2005?��ͷ����ͼ��ʾ����ƿ��ʢ������Y������Ϊ���塢��Һ����壩����Һ©����ʢ��Һ��X��U�ι���ʢ�к�īˮ�����ڹ۲죩����ʼʱa��b����ͬһˮƽ�ߣ�����X������ƿ��ʱ���ش��������⣺
��2005?��ͷ����ͼ��ʾ����ƿ��ʢ������Y������Ϊ���塢��Һ����壩����Һ©����ʢ��Һ��X��U�ι���ʢ�к�īˮ�����ڹ۲죩����ʼʱa��b����ͬһˮƽ�ߣ�����X������ƿ��ʱ���ش��������⣺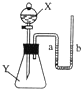 ��ͼ��ʾ����ƿ��ʢ������Y����Һ©����ʢ��Һ��X��U����ʢ�к�īˮ�����ڹ۲죩����ʼʱa��b����ͬһˮƽ�ߣ�����X������ƿ��ʱ������ش��������⣺
��ͼ��ʾ����ƿ��ʢ������Y����Һ©����ʢ��Һ��X��U����ʢ�к�īˮ�����ڹ۲죩����ʼʱa��b����ͬһˮƽ�ߣ�����X������ƿ��ʱ������ش��������⣺