��Ŀ����
����Ŀ���ʼ첿�ų����á��Ƕ���������̷۵����ӡ���ԭ���ǣ����̷��еĵ�Ԫ��ת��ΪNH3������һ������ϡ����������[2NH3��H2SO4=== (NH4)2SO4]���Ӷ�������̷��е�Ԫ�ص�����������ȷ���̷��Ƿ�ϸ�(�̷��е�Ԫ�ص����������ﵽ16%Ϊ�ϸ�)��ȡij�̷���Ʒ20 g�������еĵ�Ԫ��ת��ΪNH3����100 g������������Ϊ9.8%��ϡ����ǡ����ȫ��Ӧ��
(1)�������NH3��������
(2)ͨ�������жϸ��̷��Ƿ�ϸ�
���𰸡���1��9.8g ��2�����̷۲��ϸ�
��������(1)100 g 9.8%��ϡ�������������������Ϊ100 g��9.8 %��9.8 g
�跴Ӧ���ɰ���������Ϊx
2NH3��H2SO4=== (NH4)2SO4
34 98
x 9.8 g
![]() x��3.4 g
x��3.4 g
(2)�̷��е�Ԫ�ص���������Ϊ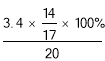 ��100 %��14 %��16 %���Ը��̷۲��ϸ�
��100 %��14 %��16 %���Ը��̷۲��ϸ�
��(1)����NH3������Ϊ3.4 g��(2)���̷۲��ϸ�

��ϰ��ϵ�д�
�����Ŀ