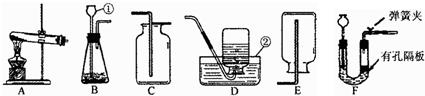
��Ŀ����
���������װ�ã��ش����⣺
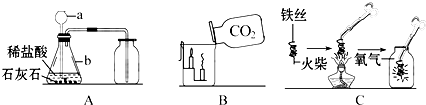
��1��д����Ţ٢ڵ��������ƣ������������������� �������������������� ��
��2��ʵ������ȡCO2�ķ�Ӧ����ʽΪ�������������������������������������������� �������ռ��������Ƿ��Ƕ�����̼��ԭ�������������������������������� ���÷���ʽ��ʾ��
��3����������غͶ������̵Ļ������ȡO2��Ĺ���������ٶ�����ȫ��Ӧ����ͨ�������IJ�ʵ������ɻ��ն������̡���ȷ�������Ⱥ�˳�������� ��������������дѡ����ţ���
?a����ɡ������� b���ܽ⡡���������� c�����ˡ��������� d��ϴ��
��4����ʵ���ҳ�ȡCO2��ʵ��װ���У�Fװ�������Bװ�þ��е��ŵ��������������������������������������������� ��
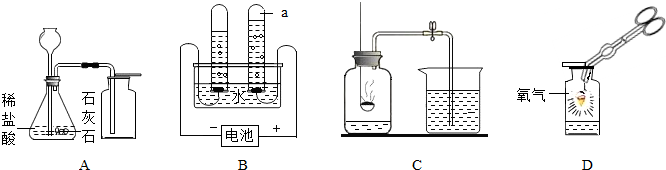
��5��ʵ����ͨ�����ַ�Ӧ���Եõ�NH3(��������ˮ���ܶȱȿ���С)�����磺
2NH4Cl(��)+Ca(OH)2(��) ![]() CaCl2+2NH3�� +2H2O �㽫ѡ����ͼ�е����� ��Ϊʵ�����ռ�NH3��װ�á���ͼ���ռ�NH3����β�����д�����װ�ã�Ũ���������NH3��Ӧ��������泥��������ͼʾ��װ�û���������ͼ��©�����ŵ������������������������������� ��
CaCl2+2NH3�� +2H2O �㽫ѡ����ͼ�е����� ��Ϊʵ�����ռ�NH3��װ�á���ͼ���ռ�NH3����β�����д�����װ�ã�Ũ���������NH3��Ӧ��������泥��������ͼʾ��װ�û���������ͼ��©�����ŵ������������������������������� ��

��1���ٳ���©�� ��ˮ��
��2��CaCO3 + 2HCl == CaCl2 + H2O + CO2�� CO2 + Ca(OH)2 = CaCO3�� + H2O��3��b��c��d��a
��4��������ʱ���Ʒ�Ӧ�ķ�����ֹͣ�������ʹ��Ӧ�濪��ͣ��
��5��E
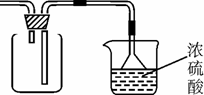
©�������ã���ֹ����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�