��Ŀ����
��ʢ��50gϡ������ձ�������ƽ�ϣ�Ȼ��ѹ�����̼���Ƽ��뵽ʢ��ϡ������ձ��У��Բⶨϡ������HCl��������������֪��Ӧǰ�ձ���ϡ�����̼���Ƶ�������Ϊ60.0g, �ӷ�Ӧ��ʼ���Ժ��6�����ڣ�ÿ1���Ӷ�1��������¼��������£�
�Իش��������⣺
��1��������һ���ⶨ������ϴ�������ڵ�ʱ���ǵ�_______min��
��2���ڸ���������ֽ�ϣ�������ʾ��Ӧ���̵�������ʱ�������ͼ��
��3���Լ���50gϡ������HCl������������д��������̣���

| ʱ��/min | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| ����/g | 60.0 | 58.0 | 57.0 | 56.8 | 56.7 | 57.2 | 56.7 |
��1��������һ���ⶨ������ϴ�������ڵ�ʱ���ǵ�_______min��
��2���ڸ���������ֽ�ϣ�������ʾ��Ӧ���̵�������ʱ�������ͼ��
��3���Լ���50gϡ������HCl������������д��������̣���
��1��5����2����ͼ����3��11%����10.95%����
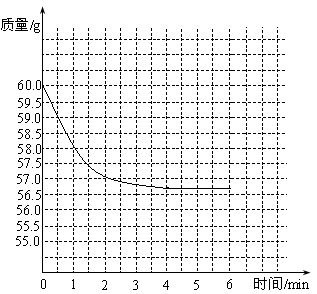

����1������̼������ϡ���ᷴӦʱ���ձ������ʵ�������Ӧ����С����ֲ��䣮���ڵ�5����ʱ�ձ�����������������˵���ⶨ������ϴ�
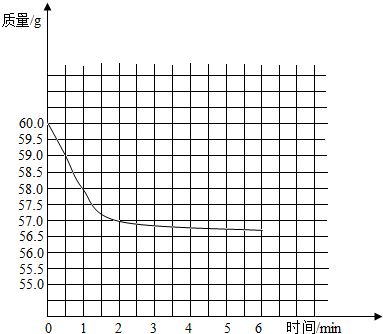
��2����ͼ
 ��
��
��3����ϡ���������ʵ���������Ϊx��
Na2CO3+2HCl=2NaCl+H2O+CO2��
73 44
50g��x 60.0g-56.7g
=
��x=10.95%
�𣺣�3��ϡ���������ʵ���������Ϊ10.95%��
��2����ͼ
 ��
����3����ϡ���������ʵ���������Ϊx��
Na2CO3+2HCl=2NaCl+H2O+CO2��
73 44
50g��x 60.0g-56.7g
| 73 |
| 44 |
| 50g��x |
| 60.0g-56.7g |
�𣺣�3��ϡ���������ʵ���������Ϊ10.95%��

��ϰ��ϵ�д�
 �ܿ�����ĩ��̾�ϵ�д�
�ܿ�����ĩ��̾�ϵ�д�
�����Ŀ

 a)g
a)g