��Ŀ����
��2012?������ģ�⣩��ѧ�����������ߣ��������ǵ�����ϢϢ��أ�H��C��O��Na��Cl��N��Fe��Ca�dz��г����ļ���Ԫ�أ���ѡ�����е�Ԫ��д����������Ҫ��Ļ�ѧ���ţ�
��1��������
��4�����Ƹ��ķ��ͷ۵�С�մ�
��6������ʯ�Ҽ����Ȼ��ƺ��Ȼ��
��7���кͷ�Ӧ
��8����������ʯ��ʯ
��9����������ɫ�����Ļ�ѧ��Ӧ
��1��������
Cl-
Cl-
��2��笠�����NH4+
NH4+
��3���ж���ҵ���ε���NaNO2
NaNO2
����4�����Ƹ��ķ��ͷ۵�С�մ�
NaHCO3
NaHCO3
��5��ʳ���к��е���CH3COOH
CH3COOH
��6������ʯ�Ҽ����Ȼ��ƺ��Ȼ��
Ca��OH��2+2NH4Cl=CaCl2+2H2O+2NH3��
Ca��OH��2+2NH4Cl=CaCl2+2H2O+2NH3��
��7���кͷ�Ӧ
NaOH+HCl=H2O+NaCl
NaOH+HCl=H2O+NaCl
��8����������ʯ��ʯ
CaCO3
CaO+CO2��
| ||
CaCO3
CaO+CO2��
| ||
��9����������ɫ�����Ļ�ѧ��Ӧ
FeCl3+3NaOH=Fe��OH��3��+3NaCl
FeCl3+3NaOH=Fe��OH��3��+3NaCl
����������ѧʽ��д��һ������ǣ���ǰ����Ȼ������ʮ�ֽ��淨����ѧ������Χ�����ֱ�ʾ��ͬ�����壺����ǰ������֣���ʾԭ�ӻ���Ӹ��������Ͻǵ����ֱ�ʾһ�����������ĵ���������½ǵ����ֱ�ʾ����ԭ�ӹ���һ�����ӣ�Ԫ�����Ϸ������ֱ�ʾԪ�صĻ��ϼۣ���д��ѧ����ʽʱ��Ҫע����ƽ��
����⣺��1��һ�������Ӵ�һ����λ�ĸ���ɣ��ʴ�Ϊ��Cl-
��2��һ��笠����Ӵ�һ����λ������ɣ��ʴ�Ϊ��NH4+
��3���ж���ҵ���ε����������ƣ���Ԫ����+1�ۣ����������-1�ۣ��ʴ�Ϊ��NaNO2
��4��С�մ���̼�����ƣ���Ԫ����+1�ۣ�̼�����-1�ۣ��ʴ�Ϊ��NaHCO3
��5��һ�����������������̼ԭ�ӡ��ĸ���ԭ�Ӻ�������ԭ�ӹ��ɵģ��ʴ�Ϊ��CH3COOH
��6����ʯ�Һ��Ȼ�立�Ӧ�����Ȼ��ƺ�ˮ�Ͱ�������ƽ���ɣ���ʯ�����Ȼ��Ʋ���Ӧ���ʴ�Ϊ��Ca��OH��2+2NH4Cl=CaCl2+2H2O+2NH3��
��7���������ƺ����ᷴӦ�����Ȼ��ƺ�ˮ���ʴ�Ϊ��NaOH+HCl=H2O+NaCl
��8��̼����ڸ��µ������£����������ƺ�ˮ���ʴ�Ϊ��CaCO3
CaO+CO2��
��9���Ȼ������������Ʒ�Ӧ�����������������ɫ�������Ȼ��ƣ���ƽ���ɣ��ʴ�Ϊ��FeCl3+3NaOH=Fe��OH��3��+3NaCl
��2��һ��笠����Ӵ�һ����λ������ɣ��ʴ�Ϊ��NH4+
��3���ж���ҵ���ε����������ƣ���Ԫ����+1�ۣ����������-1�ۣ��ʴ�Ϊ��NaNO2
��4��С�մ���̼�����ƣ���Ԫ����+1�ۣ�̼�����-1�ۣ��ʴ�Ϊ��NaHCO3
��5��һ�����������������̼ԭ�ӡ��ĸ���ԭ�Ӻ�������ԭ�ӹ��ɵģ��ʴ�Ϊ��CH3COOH
��6����ʯ�Һ��Ȼ�立�Ӧ�����Ȼ��ƺ�ˮ�Ͱ�������ƽ���ɣ���ʯ�����Ȼ��Ʋ���Ӧ���ʴ�Ϊ��Ca��OH��2+2NH4Cl=CaCl2+2H2O+2NH3��
��7���������ƺ����ᷴӦ�����Ȼ��ƺ�ˮ���ʴ�Ϊ��NaOH+HCl=H2O+NaCl
��8��̼����ڸ��µ������£����������ƺ�ˮ���ʴ�Ϊ��CaCO3
| ||
��9���Ȼ������������Ʒ�Ӧ�����������������ɫ�������Ȼ��ƣ���ƽ���ɣ��ʴ�Ϊ��FeCl3+3NaOH=Fe��OH��3��+3NaCl
�����������㿼���˻�ѧʽ����д�����ӷ��ŵ���д�ͻ�ѧ����ʽ����д��Ԫ�ط��š���ѧʽ����ѧ����ʽ�Ȼ�ѧ�������д���п�����Ҫ����֮һ����д��ѧ����ʽҪ��ƽ��Ҫ��ǿ��ϰ������Ӧ�ã���������Ҫ������ѡ�����������У�

��ϰ��ϵ�д�
�����Ŀ
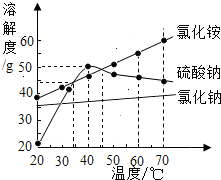 ��2012?������ģ�⣩�������ʵ��ܽ��������ͼ��������˵����ȷ���ǣ�������
��2012?������ģ�⣩�������ʵ��ܽ��������ͼ��������˵����ȷ���ǣ�������


