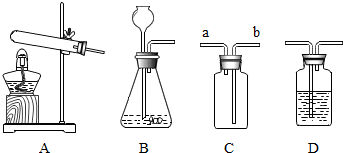
��Ŀ����
 ʵ����Ҫ����50g��������������10%���Ȼ�����Һ�������²��裺
ʵ����Ҫ����50g��������������10%���Ȼ�����Һ�������²��裺��1�����㣺��Ҫ�Ȼ��Ƶ�������
��2����������
��3���ܽ⣺��ˮ�����ձ���ͬʱ�ò�����������ʳ��ȫ���ܽ⣮�ڽ�������У���Һ����������������
��4�����ϱ�ǩ��װƿ���森
���㣺һ������������������Һ������,��������-��Ͳ,������-������ƽ
ר�⣺��Һ����Һ���ܽ��
��������1��������������=��Һ���������������������ܼ�����=��Һ������ȥ�����������㣻
��2�����ݳ���һ�����Ĺ���ѡ����ƽ����Ͳ����ѡ�����ݼ�����Ͳ��ȡһ������Һ��Ļ����������
��3�������������������ļ��㹫ʽ�жϣ�
��2�����ݳ���һ�����Ĺ���ѡ����ƽ����Ͳ����ѡ�����ݼ�����Ͳ��ȡһ������Һ��Ļ����������
��3�������������������ļ��㹫ʽ�жϣ�
����⣺��1����Ҫ�Ȼ��Ƶ�����Ϊ50g��10%=5g����Ҫˮ������Ϊ50g-5g=45g����Ҫˮ�����Ϊ45g��1g/mL=45mL��
��2��������������ƽ����Ҫˮ45mL����ѡ��50mL����Ͳ����������Ͳ�е�ˮ�ӽ��̶�ʱ���ý�ͷ�ιܵμӣ������밼Һ�����ײ�����ˮƽ��
��3���ܽ�����У�����ʳ�ε����ܽ⣬��Һ����������������ʳ��ȫ���ܽ���ٽ�����Һ���������������ı䣻
�ʴ𰸣���1��5��45��
��2����ƽ��50�����ý�ͷ�ιܵμӣ���Һ�����ײ���
��3�������ı䣻 ��
��
��2��������������ƽ����Ҫˮ45mL����ѡ��50mL����Ͳ����������Ͳ�е�ˮ�ӽ��̶�ʱ���ý�ͷ�ιܵμӣ������밼Һ�����ײ�����ˮƽ��
��3���ܽ�����У�����ʳ�ε����ܽ⣬��Һ����������������ʳ��ȫ���ܽ���ٽ�����Һ���������������ı䣻
�ʴ𰸣���1��5��45��
��2����ƽ��50�����ý�ͷ�ιܵμӣ���Һ�����ײ���
��3�������ı䣻
 ��
��������������Һ���ֳ����������������ʼ�ˮ�ܽ⣬���Ʋ������-����-�ܽ⣻Һ���ˮϡ�ͣ����Ʋ������-��ȡ-�ܽ�-װƿ��ţ�

��ϰ��ϵ�д�
 Сѧ�̲�ȫ��ϵ�д�
Сѧ�̲�ȫ��ϵ�д� Сѧ��ѧ������ѿڶ���ϵ�д�
Сѧ��ѧ������ѿڶ���ϵ�д� ������Ӧ�������������ϵ�д�
������Ӧ�������������ϵ�д� �㽭֮�ǿ�ʱ�Ż���ҵϵ�д�
�㽭֮�ǿ�ʱ�Ż���ҵϵ�д�
�����Ŀ
 ij��ѧС���ͬѧ��̼�����ƣ�NaHCO3�������ʽ���̽����
ij��ѧС���ͬѧ��̼�����ƣ�NaHCO3�������ʽ���̽����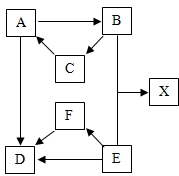 ��֪A��B��C���ֹ��庬��ͬ�ֽ���Ԫ�أ�A������ˮ��B��һ������������������D��E��F����ͬ�ַǽ���Ԫ�أ�E�dz����Ĺ��嵥�ʣ�D��Fͨ��״����Ϊ���壮B��E��Ӧ��������X��X������Ԫ����ɣ�����EԪ�ص���������Ϊ37.5%������һ��Ԫ��ԭ�Ӹ�����Ϊ2��1�����ǵ�ת����ϵ��ͼ�����ֲ�������ȥ����
��֪A��B��C���ֹ��庬��ͬ�ֽ���Ԫ�أ�A������ˮ��B��һ������������������D��E��F����ͬ�ַǽ���Ԫ�أ�E�dz����Ĺ��嵥�ʣ�D��Fͨ��״����Ϊ���壮B��E��Ӧ��������X��X������Ԫ����ɣ�����EԪ�ص���������Ϊ37.5%������һ��Ԫ��ԭ�Ӹ�����Ϊ2��1�����ǵ�ת����ϵ��ͼ�����ֲ�������ȥ����

 С�췢��������������ִ�ı����ڲ���������С�ף���ͼ��ʾ����Ϊ̽��������⣬�������ʦ�����˽������ʳƷ���������м������ɼ���̼�����ƾ���һ�ֳ��õ����ɼ���С��ӳ�������һ�����ɼ���̼�����ƣ��������о����ʵ�һ�㷽�����������̽����
С�췢��������������ִ�ı����ڲ���������С�ף���ͼ��ʾ����Ϊ̽��������⣬�������ʦ�����˽������ʳƷ���������м������ɼ���̼�����ƾ���һ�ֳ��õ����ɼ���С��ӳ�������һ�����ɼ���̼�����ƣ��������о����ʵ�һ�㷽�����������̽����