题目内容
【题目】酸、碱、盐在生产生活中具有广泛的用途。
(l)制作“叶脉书签”需用到10%的氢氧化钠溶液。现配制50g质量分数为10%的氢氧化钠溶液。
① 若用氢氧化钠固体配制,需称量氢氧化钠的质量为________g。
② 用氢氧化钠固体配制10%的氢氧化钠溶液过程中需要用到的仪器除了托盘天平、药匙、量筒、烧杯、胶头滴管、试剂瓶外,还需要________。
③ 下列操作正确的是________(填字母)。
A.称量氢氧化钠固体时,左盘放祛码
B.在托盘天平的左右托盘上垫滤纸称量氢氧化钠固体
C.将准确称量的氢氧化钠固体放入装有水的量筒中溶解
D.将配制好的氢氧化钠溶液装入试剂瓶中,塞好瓶塞并贴上标签
④若用20%的氢氧化钠溶液加水(水的密度为lg/cm3)配制50g质量分数为10%的氢氧化钠溶液,需20%的氢氧化钠溶液的质量为________g;配制时应选用________mL的量筒量取水(填“10”或“50”)。
(2)某固体粉末可能含有碳酸钙、硫酸钠、氯化钠、氯化钡、硫酸铜中的一种或几种。为确定该固体粉末的成分,进行了如下实验:
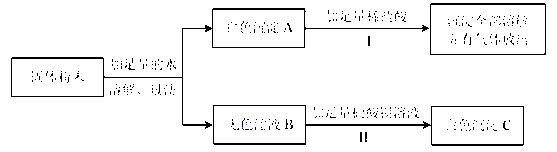
回答下列问题:
① 反应I 的化学方程式为____________。
② 白色沉淀C是________(填化学式)。
③ 原固体粉末中一定不含________(填化学式)。
【答案】(1)①5;②玻璃棒;③D;④25、50;
(2)①CaCO3+2HCl=CaCl2+CO2↑+H2O;②BaSO4;③CuSO4、BaCl2.
【解析】1)①需称量氢氧化钠的质量为50g×10%=5g;②配制溶液应该用玻璃棒搅拌,加速溶解;玻璃棒;③A.称量氢氧化钠固体时,应遵循左物右码的原则;B.氢氧化钠固体应放在烧杯中称量C.将准确称量的氢氧化钠固体放入装有水的烧杯中溶解,量筒只能用于量取一定量液体。D.将配制好的氢氧化钠溶液装入试剂瓶中,塞好瓶塞并贴上标签;④若用20%的氢氧化钠溶液加水(水的密度为lg/cm3)配制50g质量分数为10%的氢氧化钠溶液,需20%的氢氧化钠溶液的质量为5g÷20%=25g;配制时应选用50mL的量筒量取水;(2)①固体溶液水,得白色沉淀和无色溶液,说明一定没有硫酸铜,白色沉淀加入盐酸后完全溶解,并生产气体,说明沉淀是碳酸钙,碳酸钙和盐酸反应的方程式为CaCO3+2HCl=CaCl2+CO2↑+H2O;②溶液B与硝酸钡反应生成沉淀,说明固体中有硫酸钠,和硝酸钡反应生成BaSO4;同时说明固体一定没有氯化钡。

 每日10分钟口算心算速算天天练系列答案
每日10分钟口算心算速算天天练系列答案【题目】以下四个化学反应都有气体产生,其反应类型和产生的气体性质均正确的是( )
选项 | 化学反应方程式 | 反应类型 | 气体性质 |
A | Fe+H2SO4=FeSO4+H2↑ | 置换反应 | 还原性 |
B | 2H2O2 | 分解反应 | 可燃性 |
C | 2KClO3 | 化合反应 | 氧化性 |
D | CaCO3+2HCl=CaCl2+H2O+CO2↑ | 复分解反应 | 酸性 |
A、A B、B C、C D、D