��Ŀ����
С����Э����ʦ����ʵ����ʱ��������һƿ̼���ƺ��Ȼ�����ɵĻ��Һ������ⶨ����Һ��̼���ƺ��Ȼ��Ƶ�����������Ϊ����Ʋ�����������ʵ�顣
��ʵ��1��ȡ�û��Һ50 g ����������μ���ϡ���ᣬ������ϡ���������Ϊ20 g��40 g��60 g ʱ������������������±���������ܽ�Ⱥ��Բ��ƣ���
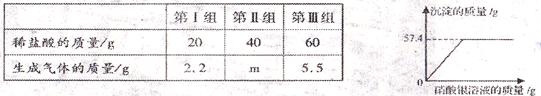
��ʵ��2����ȡ����Һ50 g ������һ����ϡ�����ַ�Ӧ�����Һ��pH=7��Ȼ���ټ�����������Һ�����ʵ�����ݼ���ͼ��
�Է������㣺
��1����ʵ��1���У��ڶ������� m Ϊ g��
��2�����Һ��̼���Ƶ����������Ƕ��٣�
��3�����Һ���Ȼ��Ƶ����������Ƕ��٣�����������ȷ��0.1%��
��ʵ��1��ȡ�û��Һ50 g ����������μ���ϡ���ᣬ������ϡ���������Ϊ20 g��40 g��60 g ʱ������������������±���������ܽ�Ⱥ��Բ��ƣ���
��ʵ��2����ȡ����Һ50 g ������һ����ϡ�����ַ�Ӧ�����Һ��pH=7��Ȼ���ټ�����������Һ�����ʵ�����ݼ���ͼ��

�Է������㣺
��1����ʵ��1���У��ڶ������� m Ϊ g��
��2�����Һ��̼���Ƶ����������Ƕ��٣�
��3�����Һ���Ȼ��Ƶ����������Ƕ��٣�����������ȷ��0.1%��
��1��4.4��1�֣� ��2��26.5% ��3��17.5%
���ݱ������ݷ������ڢ�������ϡ������ȫ��Ӧ����ÿ20g��ȫ��Ӧ����������̼������Ϊ2.2g���ݴ˿��ж�m=4.4���ҵڢ������������������Ʒ��ȫ��Ӧ������������Ϊ5.5g�����ݶ�����̼�������ɼ�������Һ��̼���Ƶ��������������������Ӧ�����Ȼ��Ƶ����������ݷ�Ӧ��Ļ����Һ����������Ӧ��ͼ��ɼ������Ӧ����Һ���Ȼ��Ƶ�����������Ӧ����Һ���Ȼ���������ȥ��Ӧ���ɵ��Ȼ��Ƶ���������ԭ���Һ���Ȼ��Ƶ��������ݴ˿ɼ����ԭ���Һ���Ȼ��Ƶ�����������
��2���⣺�������ݿ�֪50 g�����Һ��̼������������ȫ��Ӧ����5.5g������̼��
��50 g�����Һ��̼��������Ϊx��ͬʱ�����ᷴӦ�����Ȼ��Ƶ�����Ϊy��
Na2CO3 + 2HCl =" 2NaCl" + H2O + CO2����1�֣�
106 117 44
x y 5.5g
106/44=x/5.5g x=13.25g��1�֣�
117/44=y/5.5g y=14.63g��1�֣�
�����Һ��̼���Ƶ�����������13.25g/50g��100��=26.5����1�֣�
��3���������Ȼ���������Ҫ�Ȼ��Ƶ�����Ϊz
NaCl + AgNO3 = AgCl �� + NaNO3��1�֣�
58.5 143.5
z 57.4g
58.5/143.5=z/57.4g z=23.4g��1�֣�
�����Һ���Ȼ��Ƶ���������Ϊ��(23.4g��14.63g)/50g��100��=17.5����1�֣�
�𣺻��Һ��̼���Ƶ���������Ϊ26.5�������Һ���Ȼ��Ƶ���������Ϊ17.5����
��2���⣺�������ݿ�֪50 g�����Һ��̼������������ȫ��Ӧ����5.5g������̼��
��50 g�����Һ��̼��������Ϊx��ͬʱ�����ᷴӦ�����Ȼ��Ƶ�����Ϊy��
Na2CO3 + 2HCl =" 2NaCl" + H2O + CO2����1�֣�
106 117 44
x y 5.5g
106/44=x/5.5g x=13.25g��1�֣�
117/44=y/5.5g y=14.63g��1�֣�
�����Һ��̼���Ƶ�����������13.25g/50g��100��=26.5����1�֣�
��3���������Ȼ���������Ҫ�Ȼ��Ƶ�����Ϊz
NaCl + AgNO3 = AgCl �� + NaNO3��1�֣�
58.5 143.5
z 57.4g
58.5/143.5=z/57.4g z=23.4g��1�֣�
�����Һ���Ȼ��Ƶ���������Ϊ��(23.4g��14.63g)/50g��100��=17.5����1�֣�
�𣺻��Һ��̼���Ƶ���������Ϊ26.5�������Һ���Ȼ��Ƶ���������Ϊ17.5����

��ϰ��ϵ�д�
�����Ŀ

