��Ŀ����
����Ŀ����ѧ������ϢϢ��أ���������ػ�ѧ֪ʶ���ͣ�
��1�����������ȵ�����ȿ��֣�����CO2��������ϣ�������"����"��������Ϊ������ܽ�����¶ȵ����߶�_____����"��������"����
��2������д�������з�ֹ����Ʒ�����һ�־��巽��_____��
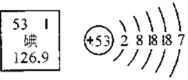
��3��"XXѩ��"��װ���ڸ��������Ҫ�ɷ�����ʯ�ң���ѧʽCaO�����������ʯ������������������ǣ��û�ѧ����ʽ��ʾ��_____��
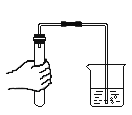
��4��ҽ����ҩˮ��������������Ϊ0.1%��KMnO4��Һ��������2000g��ҩˮ�����ȡKMnO4��������_____�ˡ�
���𰸡���С ˢ���ᣨ�𰸺������ɣ� CaO+H2O=Ca(OH)2 2
��������
�¶����ߣ�������ܽ�ȼ�С. ѹǿ����������ܽ�������������ǽ�����������ˮ��ͬ���õĽ���������ƺ�ˮ��Ӧ�����������ơ�
��1���¶����ߣ�������ܽ�ȼ�С. ѹǿ����������ܽ�����ʳ�����"����"��������Ϊ������ܽ�����¶ȵ����߶����١�
��2��������������������ˮ��ͬ���õĽ�����ʷ�ֹ����Ʒ�������ˢ���ᡣ
��3����ʯ�ң���ѧʽCaO����ˮ��Ӧ�����������ƣ�����ʯ������������Ļ�ѧ����ʽΪ![]() ��
��
��4��ҽ����ҩˮ��������������Ϊ0.1%��KMnO4��Һ��������2000g��ҩˮ�����ȡKMnO4��������Ϊ![]() ��
��

 Ӧ�����������Ĵ���ѧ������ϵ�д�
Ӧ�����������Ĵ���ѧ������ϵ�д�����Ŀ��Ϊ�ⶨij����ͭ��ͭ�Ĺ�������������ͭ������������С��ͬѧȡ20g�������������ձ��У���100gϡ�����Ϊ�ĵȷ����μ������н���ʵ�飬����������£�
���� | �� | �� | �� | �� |
����ϡ���������/g | 25 | 25 | 25 | 25 |
ʣ����������/g | 16 | a | 10 | 10 |
�ش������⣺
��1��ԭ���������У�����ͭ����������Ϊ_____��
��2���ϱ��У�a��ֵΪ_____��ʵ����������Һ�е�������_____���ѧʽ����
��3����ʵ������ϡ���������ʵ���������Ϊ____����д��������̣������ȷ��0.1%��
����Ŀ��С���ȡ5.0gijƷ�ƻ���(�̱���ͼ1)���ձ��У���ˮ��ȫ�ܽ⣬����Ba(OH)2��Һ���иû�����(NH4)2SO4�����IJⶨ(��������ˮ�����μӷ�Ӧ)���ⶨ���������ͼ2��ʾ��
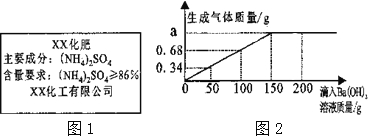
����Ba(OH)2��Һ����/g | 50 | 100 | 150 | 200 |
���ɳ�������/g | 2.33 | 4.66 | 6.99 | m |
��֪��(NH4)2SO4+Ba(OH)2�TBaSO4��+2NH3��+2H2O
(1)�����е�m�������ϵ�a��ֵ�ֱ�Ϊ ____��____��
(2)�û�������____(�����ϸ����������ϸ���)��Ʒ
(3)��������Ba(OH)2��Һ�����ʵ���������____(д��������̣������ȷ��0.01%)��