��Ŀ����
����Ŀ����������( Na2FeO4)��һ��������ɫ������,��Ҫ��������ˮ������ij������22.35 kg NaClO���������Fe2(SO4)3�������������Ϊ20%��NaOH��ҺΪԭ������ Na2FeO4,��Ӧԭ��Ϊ:3NaClO+Fe2(SO4)3+10NaOH = 2Na2FeO4+3NaCl+3Na2SO4+5H2O�����跴Ӧ���ǡ����ȫ��Ӧ���Լ���:
[��֪:��Է�������Ϊ NaClO 74.5 Fe2(SO4)3 400 Na2FeO4 166]
��1��Na2FeO4��������������Ԫ����_____
��2���Ʊ������������20%��NaOH��Һ�������Ƕ���?_____
��3�����������Һ��Na2FeO4�����������Ƕ���?_____(��������ȷ��0.1%)
���𰸡���Ԫ�� 200kg 12.7%
��������
��1��Na2FeO4���ơ�������Ԫ��������=��23��2����56����16��4��=23��28��32����������������������Ԫ�أ�
��2���裺NaOH������Ϊx��
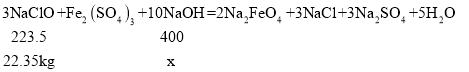
![]() x=40kg
x=40kg
20%��NaOH��Һ������=40kg��20%=200kg��
��:����20%��NaOH��Һ��������200kg��
��3����:��Fe2(SO4)3������Ϊy�� Na2FeO4������z
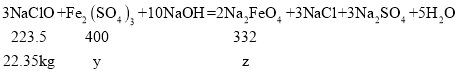
![]() y=40kg��
y=40kg��
![]() z=33.2g
z=33.2g
���������Һ��Na2FeO4����������=![]()
�����������Һ��Na2FeO4������������12.7%��