��Ŀ����
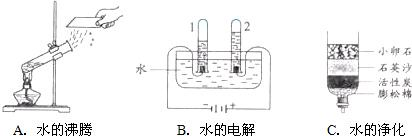
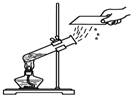
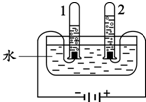
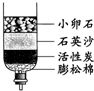
��1����ͼ��ʾ��3��ʵ�飬A��ˮ������
��2����Ȫˮ������ˮ�ж������ã����磺þ���Ļ��Ϸ�Ӧʮ�ֻ��������μ�����ˮ����Ӧ�������ҽ��У���Ӧ�ٶȴ����ߣ���ʱˮ��������
��3����Լ��ˮ��ÿ�����������������ˮ��ʽӦ���ᳫ����
A�������ڱ���ˮˢ����B������ϵر���ˮ��ϴ��
C��������ˮ��ϴ��ˮ�������������D������ࡢ�ι�ķ�������ũ�����
��4��ClO2����һ������ˮ����������������������Cl2��������ˮ����������ȡClO2�ķ�Ӧ����ʾ��ͼ���£���ش�
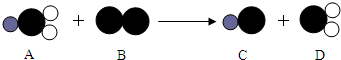
�����У� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�C��������Ԫ�صĻ��ϼ���
��ʾ��ԭ�ӣ�C��������Ԫ�صĻ��ϼ���
����
����
�����������ѧ�����仯��B���Թ�2�ڵõ�������Ϊ������O2
������O2
����ʵ��˵��ˮ�����⡢��Ԫ��
�⡢��Ԫ��
��ɵģ��÷�Ӧ�Ļ�ѧ����ʽ��2H2O
2H2��+O2��
| ||
2H2O
2H2��+O2��
��C��С��ʯ��ʯӢɳ��������������
| ||
����
����
�������˾�ˮ���õ���ˮ��Ȼ���Ǵ�ˮ������õ���ˮ�ɲ��õķ���������
����
�� |
 |
 |
| A��ˮ�ķ��� | B��ˮ�ĵ�� | C��ˮ�ľ��� |
������
������
����3����Լ��ˮ��ÿ�����������������ˮ��ʽӦ���ᳫ����
ACD
ACD
������ĸ��ţ���A�������ڱ���ˮˢ����B������ϵر���ˮ��ϴ��
C��������ˮ��ϴ��ˮ�������������D������ࡢ�ι�ķ�������ũ�����
��4��ClO2����һ������ˮ����������������������Cl2��������ˮ����������ȡClO2�ķ�Ӧ����ʾ��ͼ���£���ش�

������
 ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�C��������Ԫ�صĻ��ϼ���
��ʾ��ԭ�ӣ�C��������Ԫ�صĻ��ϼ���-1
-1
��D���ʵ���������������
��������
���÷�Ӧ�Ļ�ѧ����ʽ��2NaClO2+Cl2�T2NaCl+2ClO2
2NaClO2+Cl2�T2NaCl+2ClO2
��������C��D������֮��13��15
13��15
����������1�����ݵ��ˮ������ͽ��ۡ�ˮ�ľ����������Ƿ����������ʣ���ϸ�������н��
��2�����ݴ����ܸı��������ʵĻ�ѧ��Ӧ���ʣ��������������ͻ�ѧ���ʲ�������жϣ�
��3�����������г��ý�ˮ�����жϣ�
��4����ͼ�Ʋ�C����Ϊ�Ȼ��ƣ���֪��Ԫ�صĻ��ϼ�Ϊ+1�ۣ��ʿ�֪��Ԫ�صĻ��ϼۣ���֪�� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ����֪D���ʵ����ƣ����Ʋ�A��B��C��D�Ļ�ѧʽ������д�÷�Ӧ�Ļ�ѧ����ʽ�����ݻ�ѧ����ʽ������ʵ������ȣ�
��ʾ��ԭ�ӣ����֪D���ʵ����ƣ����Ʋ�A��B��C��D�Ļ�ѧʽ������д�÷�Ӧ�Ļ�ѧ����ʽ�����ݻ�ѧ����ʽ������ʵ������ȣ�
��2�����ݴ����ܸı��������ʵĻ�ѧ��Ӧ���ʣ��������������ͻ�ѧ���ʲ�������жϣ�
��3�����������г��ý�ˮ�����жϣ�
��4����ͼ�Ʋ�C����Ϊ�Ȼ��ƣ���֪��Ԫ�صĻ��ϼ�Ϊ+1�ۣ��ʿ�֪��Ԫ�صĻ��ϼۣ���֪��
 ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ����֪D���ʵ����ƣ����Ʋ�A��B��C��D�Ļ�ѧʽ������д�÷�Ӧ�Ļ�ѧ����ʽ�����ݻ�ѧ����ʽ������ʵ������ȣ�
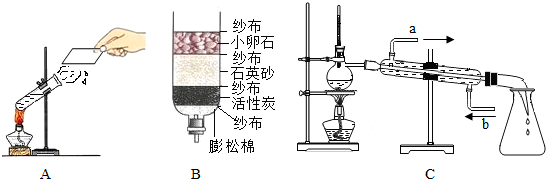
��ʾ��ԭ�ӣ����֪D���ʵ����ƣ����Ʋ�A��B��C��D�Ļ�ѧʽ������д�÷�Ӧ�Ļ�ѧ����ʽ�����ݻ�ѧ����ʽ������ʵ������ȣ�����⣺��1��ͼA��ˮ�ķ��ڣ�ˮ��Һ�������壬û�������������ʣ���˸ñ仯�������仯��Bͼ�ǵ��ˮװ��ͼ���ڵ��ˮ�У��ɿ����������ǣ����������٣���ʹ�����ǵ�ľ������ȼ������������������࣬��ȼ�գ������������������ٵĶ�����ͨ��ʵ�黹�ܽ�һ���Ƴ�ˮ����Ԫ�غ���Ԫ����ɣ��Թ�1�������������Թ�2���ռ���������������ͼC�����ƾ�ˮ����������ʯ��ʯӢɰ����������ֽ����һ�����������ã�������ͨ�����ȵķ���ʹˮ���ˮ��������������ˮ���ɵõ���ˮ��
�ʴ�Ϊ��
������������O2���⡢��Ԫ�أ�2H2O
2H2��+O2�������ˣ�����
��2���μ�����ˮ����Ӧ�������ҽ��У���Ӧ�ٶȴ����ߣ����ϴ������ص㣬ˮ���˴������ã�
�ʴ�Ϊ�������ã�
��3������Ӧ����ϧÿһ��ˮ����������Ӧ���ᳫ�������ڱ���ˮˢ����������ˮ��ϴ��ˮ�����������������ࡢ�ι�ķ�������ũ����֣���ѡACD��
��ͼ�Ʋ�C����Ϊ�Ȼ��ƣ���֪��Ԫ�صĻ��ϼ�Ϊ+1�ۣ����ݻ��������������ϼ��ܺ�Ϊ0����֪��Ԫ�صĻ��ϼ���-1����ͼ��֪D���ʵ������Ƕ������ȣ�
A��B��C��D�Ļ�ѧʽ�ֱ�Ϊ��NaClO2��Cl2��NaCl��ClO2���ʸ÷�Ӧ�Ļ�ѧ����ʽ��2NaClO2+Cl2�T2NaCl+2ClO2��
�ɷ���ʽ��֪��C��D��������Ϊ��117��135=13��15��
�ʴ�Ϊ��-1���������ȣ�2NaClO2+Cl2�T2NaCl+2ClO2��13��15��
�ʴ�Ϊ��
������������O2���⡢��Ԫ�أ�2H2O
| ||
��2���μ�����ˮ����Ӧ�������ҽ��У���Ӧ�ٶȴ����ߣ����ϴ������ص㣬ˮ���˴������ã�
�ʴ�Ϊ�������ã�
��3������Ӧ����ϧÿһ��ˮ����������Ӧ���ᳫ�������ڱ���ˮˢ����������ˮ��ϴ��ˮ�����������������ࡢ�ι�ķ�������ũ����֣���ѡACD��
��ͼ�Ʋ�C����Ϊ�Ȼ��ƣ���֪��Ԫ�صĻ��ϼ�Ϊ+1�ۣ����ݻ��������������ϼ��ܺ�Ϊ0����֪��Ԫ�صĻ��ϼ���-1����ͼ��֪D���ʵ������Ƕ������ȣ�
A��B��C��D�Ļ�ѧʽ�ֱ�Ϊ��NaClO2��Cl2��NaCl��ClO2���ʸ÷�Ӧ�Ļ�ѧ����ʽ��2NaClO2+Cl2�T2NaCl+2ClO2��
�ɷ���ʽ��֪��C��D��������Ϊ��117��135=13��15��
�ʴ�Ϊ��-1���������ȣ�2NaClO2+Cl2�T2NaCl+2ClO2��13��15��
���������⿼��֪ʶ��Ƚ϶࣬Ҫ������ѧϰ��֪ʶ�����о����ȫ��ķ������������

��ϰ��ϵ�д�
�����Ŀ