��Ŀ����
��2013?�Ͼ�������Ӧ����㷺�Ľ�����
��1��д����˿��������ȼ�յĻ�ѧ����ʽ
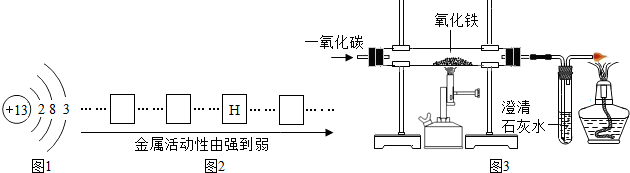
��2�����Ͳ�������Fe����������Ч������ʵ���Ҳ��û�ԭ���Ʊ�����Fe�ۣ���������ͼ��ʾ��
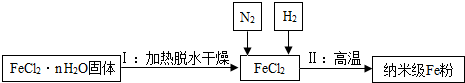
������Fe���ڿ���������ȼ��ʵ����ͨ��N2��Ŀ����
��д������H2��ԭFeCl2�û������Ļ�ѧ����ʽ
��1��д����˿��������ȼ�յĻ�ѧ����ʽ
3Fe+2O2
Fe3O4
| ||
3Fe+2O2
Fe3O4
��
| ||
��2�����Ͳ�������Fe����������Ч������ʵ���Ҳ��û�ԭ���Ʊ�����Fe�ۣ���������ͼ��ʾ��

������Fe���ڿ���������ȼ��ʵ����ͨ��N2��Ŀ����
��ֹ���ɵ��������۱�����
��ֹ���ɵ��������۱�����
����д������H2��ԭFeCl2�û������Ļ�ѧ����ʽ
H2+FeCl2
Fe+2HCl
| ||
H2+FeCl2
Fe+2HCl
��
| ||
��������1���������ڴ�����ȼ�������������������н��
��2��������ɿ�֪����������Fe�������������»�ԭ�Ȼ������õ��ģ��Լ��ڸ��������ɵ����ױ���������������Ҫʹ�ñ�������
��2��������ɿ�֪����������Fe�������������»�ԭ�Ȼ������õ��ģ��Լ��ڸ��������ɵ����ױ���������������Ҫʹ�ñ�������
����⣺��1�����ڴ�����ȼ��������������������Ӧ�Ļ�ѧ����ʽ��3Fe+2O2
Fe3O4��
��2���ڸ������������ɵ����ױ���������������Ҫʹ�ñ���������ֹ����������е������Ӵ���ȼ���ڸ��µ������£�H2��FeCl2��Ӧ���������������ۺ��Ȼ������壬��Ӧ�ķ���ʽΪ��H2+FeCl2
Fe+2HCl��
�ʴ�Ϊ����1��3Fe+2O2
Fe3O4��
��2����ֹ���ɵ��������۱�������H2+FeCl2
Fe+2HCl��
| ||
��2���ڸ������������ɵ����ױ���������������Ҫʹ�ñ���������ֹ����������е������Ӵ���ȼ���ڸ��µ������£�H2��FeCl2��Ӧ���������������ۺ��Ȼ������壬��Ӧ�ķ���ʽΪ��H2+FeCl2
| ||
�ʴ�Ϊ����1��3Fe+2O2
| ||
��2����ֹ���ɵ��������۱�������H2+FeCl2
| ||
���������⿼���˽��������й����ʣ���ɴ��⣬���Խ������ṩ����Ϣ����ѧ�������й����ʽ��н��

��ϰ��ϵ�д�
�����Ŀ