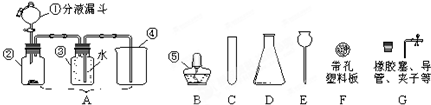
��Ŀ����
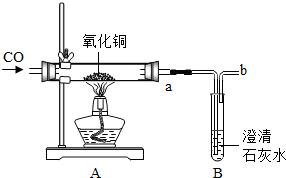 ijС�齫һ������������ͭ��ĩ������ͼ��װ���н���ʵ�飮������װ�ô��ڵ����������Ϊ����װ�õľ��巽����
ijС�齫һ������������ͭ��ĩ������ͼ��װ���н���ʵ�飮������װ�ô��ڵ����������Ϊ����װ�õľ��巽�����Թܿڼ���Ƥ����b����ȼ
�Թܿڼ���Ƥ����b����ȼ
��ʵ�鿪ʼʱΪ���ų��Թ���ʣ��Ŀ���Ӧ��ͨһ����̼�����
��ͨһ����̼�����
��������Ӳ�ʲ����ܣ���A���й���ȫ����Ϊ��ɫ
Ӳ�ʲ����ܣ���A���й���ȫ����Ϊ��ɫ
ʱ֤������ͭ����ȫת������ͭ����ʱ������Ӧ����ȷ�IJ���˳������ֹͣ���Ⱥ�ֹͣͨһ����̼
��ֹͣ���Ⱥ�ֹͣͨһ����̼
����ȴ�����ʣ������������6.4g�����ж���Ħ��һ����̼�μ��˷�Ӧ������������һ����̼�ǶԴ�������Ⱦ�����壬��������װ�õľ��巽��������һ����̼�ǿ�ȼ�Ե����壬������Ļ�������ڵ�ȼʱ�ױ�ը������ʼʱ�IJ�������������ͭ��ͭ����ɫ�IJ�ͬ��������Ӧ���еij̶ȣ����ݻ�ԭ�����ȵ�ͭ���ڿ����е�������Ӧ��������Ӧ����ʱ�IJ���������һ����̼��ԭ����ͭ�ķ���ʽ����ͭ����������μ��˷�Ӧһ����̼�����ʵ�����
����⣺����һ����̼�Դ�������Ⱦ����������װ�õľ��巽�����Թܿڼ���Ƥ����b����ȼ������һ����̼�ǿ�ȼ�Ե����壬������Ļ�������ڵ�ȼʱ�ױ�ը�����ԣ���ʼʱΪ���ų��Թ���ʣ��Ŀ���Ӧ��ͨһ����̼����ȣ�������Ӳ�ʲ������й���ȫ����Ϊ��ɫʱ��֤������ͭ����ȫת������ͭ�����ڸջ�ԭ����ͭ���ȵģ����ڿ����е�������Ӧ��������������ͭ�����ԣ���Ӧ����ʱ�IJ����ǣ���ֹͣ���Ⱥ�ֹͣͨһ����̼��
��μӷ�Ӧ��һ����̼�����ʵ���Ϊx��
����ͭ�����ʵ���Ϊ��
=0.1mol
CO+CuO
Cu+CO2
1 1
X 0.1mol
=
X=0.1mol
�ʴ�Ϊ���Թܿڼ���Ƥ����b����ȼ����ͨһ����̼����ȣ�Ӳ�ʲ������й���ȫ����Ϊ��ɫ����ֹͣ���Ⱥ�ֹͣͨһ����̼����0.1mol��һ����̼�μ��˷�Ӧ��
��μӷ�Ӧ��һ����̼�����ʵ���Ϊx��
����ͭ�����ʵ���Ϊ��
| 6.4g |
| 64g/mol |
CO+CuO
| ||
1 1
X 0.1mol
| 1 |
| 1 |
| x |
| 0.1mol |
�ʴ�Ϊ���Թܿڼ���Ƥ����b����ȼ����ͨһ����̼����ȣ�Ӳ�ʲ������й���ȫ����Ϊ��ɫ����ֹͣ���Ⱥ�ֹͣͨһ����̼����0.1mol��һ����̼�μ��˷�Ӧ��
������������Ҫ������һ����̼��ԭ����ͭ��ʵ�飮Ҫ��ɺñ��⣬��Ҫȫ�������һ����̼�Ŀ�ȼ�ԡ��Դ�������Ⱦ��һ����̼��ԭ����ͭ�IJ������輰ע��������֪ʶ��

��ϰ��ϵ�д�
 ���ɿ��ñ���ϵ�д�
���ɿ��ñ���ϵ�д�
�����Ŀ
 ��1��ʱ����Щ������ϲ��Ⱦ����Ⱦ��ʱһ��Ҫ�õ�һ����ɫ��--�Ա��������仯ѧʽΪC6H8N2������һ���ж��Ļ�ѧҩƷ�����Ⱦ���ߵ���������˺���
��1��ʱ����Щ������ϲ��Ⱦ����Ⱦ��ʱһ��Ҫ�õ�һ����ɫ��--�Ա��������仯ѧʽΪC6H8N2������һ���ж��Ļ�ѧҩƷ�����Ⱦ���ߵ���������˺���
�ٶԱ���������Է�������Ϊ______��
��108g�Ա������к���Ԫ�ص�����Ϊ______��
��2��ij�о���ѧϰС���һ��NaHCO3��KHCO3�Ļ������Ʒ����������̽�����밴Ҫ���������̽�����棬��ʾ��
2NaHCO3+H2SO4=Na2SO4+2H2O+2CO2��
2KHCO3+H2SO4=K2SO4+2H2O+2CO2��
̽��Ŀ�ģ�ʵ��ⶨ��Ʒ��NaHCO3��KHCO3��������֮�ȣ�
̽��˼·�����ʵ������йط�Ӧ����������������ͨ������ȷ����Ʒ��NaHCO3��KHCO3��������֮�ȣ�
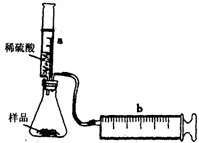
ʵ��̽����ʵ��װ����ͼ��ʾ����һ�������Ļ������Ʒ������ƿ�У�����Ͳaע��һ�������ϡ�����ַ�Ӧ��������Ͳb�ռ���������������������Ͳ�ܱڼ��Ħ�������������ƿ��ע���ϡ��������ΪV1mL����ַ�Ӧ����Ͳb������Ķ���ΪV2mL����Ӧ�����ɵ�CO2�������ԼΪ______mL��
�����������ƿ�з��л�Ͼ��ȵ�NaHCO3��KHCO3����Ʒ3.6g������ƿ�е���һ������ϡ���ᣬ���ɵ������������Ѿ���������㣩������ϡ����������ϵ���±���ʾ��
| ����������mL�� | 5 | 10 | 15 | 20 | 25 |
| ������̼��������g�� | 0.44 | 0.88 | a | 1.76 | 1.76 |
��NaHCO3��KHCO3�Ļ������Ʒ����Ԫ�ص���������______���������ȷ��0.1%��
����Ʒ��NaHCO3��KHCO3���ʵ�����֮��Ϊ______��
��1��ʱ����Щ������ϲ��Ⱦ����Ⱦ��ʱһ��Ҫ�õ�һ����ɫ��--�Ա��������仯ѧʽΪC6H8N2������һ���ж��Ļ�ѧҩƷ�����Ⱦ���ߵ���������˺���
�ٶԱ���������Է�������Ϊ______��
��108g�Ա������к���Ԫ�ص�����Ϊ______��
��2��ij�о���ѧϰС���һ��NaHCO3��KHCO3�Ļ������Ʒ����������̽�����밴Ҫ���������̽�����棬��ʾ��
2NaHCO3+H2SO4=Na2SO4+2H2O+2CO2��
2KHCO3+H2SO4=K2SO4+2H2O+2CO2��
̽��Ŀ�ģ�ʵ��ⶨ��Ʒ��NaHCO3��KHCO3��������֮�ȣ�
̽��˼·�����ʵ������йط�Ӧ����������������ͨ������ȷ����Ʒ��NaHCO3��KHCO3��������֮�ȣ�
ʵ��̽����ʵ��װ����ͼ��ʾ����һ�������Ļ������Ʒ������ƿ�У�����Ͳaע��һ�������ϡ�����ַ�Ӧ��������Ͳb�ռ���������������������Ͳ�ܱڼ��Ħ�������������ƿ��ע���ϡ��������ΪV1mL����ַ�Ӧ����Ͳb������Ķ���ΪV2mL����Ӧ�����ɵ�CO2�������ԼΪ______mL��
�����������ƿ�з��л�Ͼ��ȵ�NaHCO3��KHCO3����Ʒ3.6g������ƿ�е���һ������ϡ���ᣬ���ɵ������������Ѿ���������㣩������ϡ����������ϵ���±���ʾ��
��a=______g
��NaHCO3��KHCO3�Ļ������Ʒ����Ԫ�ص���������______���������ȷ��0.1%��
����Ʒ��NaHCO3��KHCO3���ʵ�����֮��Ϊ______��

�ٶԱ���������Է�������Ϊ______��
��108g�Ա������к���Ԫ�ص�����Ϊ______��
��2��ij�о���ѧϰС���һ��NaHCO3��KHCO3�Ļ������Ʒ����������̽�����밴Ҫ���������̽�����棬��ʾ��
2NaHCO3+H2SO4=Na2SO4+2H2O+2CO2��
2KHCO3+H2SO4=K2SO4+2H2O+2CO2��
̽��Ŀ�ģ�ʵ��ⶨ��Ʒ��NaHCO3��KHCO3��������֮�ȣ�
̽��˼·�����ʵ������йط�Ӧ����������������ͨ������ȷ����Ʒ��NaHCO3��KHCO3��������֮�ȣ�
ʵ��̽����ʵ��װ����ͼ��ʾ����һ�������Ļ������Ʒ������ƿ�У�����Ͳaע��һ�������ϡ�����ַ�Ӧ��������Ͳb�ռ���������������������Ͳ�ܱڼ��Ħ�������������ƿ��ע���ϡ��������ΪV1mL����ַ�Ӧ����Ͳb������Ķ���ΪV2mL����Ӧ�����ɵ�CO2�������ԼΪ______mL��
�����������ƿ�з��л�Ͼ��ȵ�NaHCO3��KHCO3����Ʒ3.6g������ƿ�е���һ������ϡ���ᣬ���ɵ������������Ѿ���������㣩������ϡ����������ϵ���±���ʾ��
| ����������mL�� | 5 | 10 | 15 | 20 | 25 |
| ������̼��������g�� | 0.44 | 0.88 | a | 1.76 | 1.76 |
��NaHCO3��KHCO3�Ļ������Ʒ����Ԫ�ص���������______���������ȷ��0.1%��
����Ʒ��NaHCO3��KHCO3���ʵ�����֮��Ϊ______��

��1��ʱ����Щ������ϲ��Ⱦ����Ⱦ��ʱһ��Ҫ�õ�һ����ɫ��--�Ա��������仯ѧʽΪC6H8N2������һ���ж��Ļ�ѧҩƷ�����Ⱦ���ߵ���������˺���
�ٶԱ���������Է�������Ϊ______��
��108g�Ա������к���Ԫ�ص�����Ϊ______��
��2��ij�о���ѧϰС���һ��NaHCO3��KHCO3�Ļ������Ʒ����������̽�����밴Ҫ���������̽�����棬��ʾ��
2NaHCO3+H2SO4=Na2SO4+2H2O+2CO2��
2KHCO3+H2SO4=K2SO4+2H2O+2CO2��
̽��Ŀ�ģ�ʵ��ⶨ��Ʒ��NaHCO3��KHCO3��������֮�ȣ�
̽��˼·�����ʵ������йط�Ӧ����������������ͨ������ȷ����Ʒ��NaHCO3��KHCO3��������֮�ȣ�
ʵ��̽����ʵ��װ����ͼ��ʾ����һ�������Ļ������Ʒ������ƿ�У�����Ͳaע��һ�������ϡ�����ַ�Ӧ��������Ͳb�ռ���������������������Ͳ�ܱڼ��Ħ�������������ƿ��ע���ϡ��������ΪV1mL����ַ�Ӧ����Ͳb������Ķ���ΪV2mL����Ӧ�����ɵ�CO2�������ԼΪ______mL��
�����������ƿ�з��л�Ͼ��ȵ�NaHCO3��KHCO3����Ʒ3.6g������ƿ�е���һ������ϡ���ᣬ���ɵ������������Ѿ���������㣩������ϡ����������ϵ���±���ʾ��
��a=______g
��NaHCO3��KHCO3�Ļ������Ʒ����Ԫ�ص���������______���������ȷ��0.1%��
����Ʒ��NaHCO3��KHCO3���ʵ�����֮��Ϊ______��
�ٶԱ���������Է�������Ϊ______��
��108g�Ա������к���Ԫ�ص�����Ϊ______��
��2��ij�о���ѧϰС���һ��NaHCO3��KHCO3�Ļ������Ʒ����������̽�����밴Ҫ���������̽�����棬��ʾ��
2NaHCO3+H2SO4=Na2SO4+2H2O+2CO2��
2KHCO3+H2SO4=K2SO4+2H2O+2CO2��
̽��Ŀ�ģ�ʵ��ⶨ��Ʒ��NaHCO3��KHCO3��������֮�ȣ�
̽��˼·�����ʵ������йط�Ӧ����������������ͨ������ȷ����Ʒ��NaHCO3��KHCO3��������֮�ȣ�
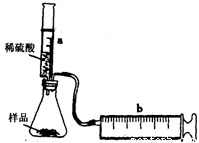
ʵ��̽����ʵ��װ����ͼ��ʾ����һ�������Ļ������Ʒ������ƿ�У�����Ͳaע��һ�������ϡ�����ַ�Ӧ��������Ͳb�ռ���������������������Ͳ�ܱڼ��Ħ�������������ƿ��ע���ϡ��������ΪV1mL����ַ�Ӧ����Ͳb������Ķ���ΪV2mL����Ӧ�����ɵ�CO2�������ԼΪ______mL��
�����������ƿ�з��л�Ͼ��ȵ�NaHCO3��KHCO3����Ʒ3.6g������ƿ�е���һ������ϡ���ᣬ���ɵ������������Ѿ���������㣩������ϡ����������ϵ���±���ʾ��
| ����������mL�� | 5 | 10 | 15 | 20 | 25 |
| ������̼��������g�� | 0.44 | 0.88 | a | 1.76 | 1.76 |
��NaHCO3��KHCO3�Ļ������Ʒ����Ԫ�ص���������______���������ȷ��0.1%��
����Ʒ��NaHCO3��KHCO3���ʵ�����֮��Ϊ______��

 ��1��ʱ����Щ������ϲ��Ⱦ����Ⱦ��ʱһ��Ҫ�õ�һ����ɫ��--�Ա��������仯ѧʽΪC6H8N2������һ���ж��Ļ�ѧҩƷ�����Ⱦ���ߵ���������˺���
��1��ʱ����Щ������ϲ��Ⱦ����Ⱦ��ʱһ��Ҫ�õ�һ����ɫ��--�Ա��������仯ѧʽΪC6H8N2������һ���ж��Ļ�ѧҩƷ�����Ⱦ���ߵ���������˺���