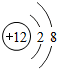��Ŀ����
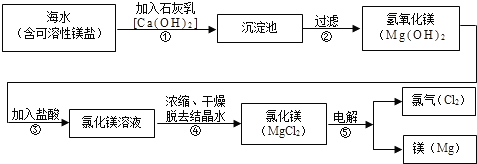
þ�Ͻ��ǿ�ȸߡ���е���ܺã���Щ����ʹ����þ��Ϊ�����������ɻ����������Ҫ���ϣ��Ӷ���á���������������������ˮ��þ�ǹ����ϵ���Ҫ���ƣ���ҵ����ͼ���£�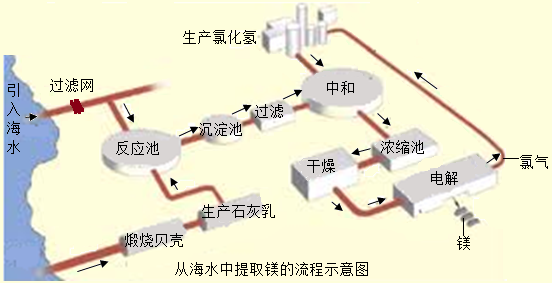
�䲽��Ϊ��
I�������ߴ������ڵı��ǣ���Ҫ�ɷ�ΪCaCO3���ճ���ʯ�ң�������ʯ���Ƴ�ʯ���飻
��ʯ������뵽��ˮ��Ӧ���У��������������˵õ�Mg��OH��2������
����Mg��OH��2�����м��������к͵õ�MgCl2��Һ���پ������ᾧ�õ�MgCl2?6H2O��
������MgCl2?6H2O��һ�������¼��ȵõ���ˮMgCl2��
V��������ڵ���ˮMgCl2�ɵõ�Mg��1���������������ش����⣺
��д������I������йػ�ѧ����ʽ��
I���������ճ���ʯ��
��Mg��OH��2�����м��������к�
�ڲ���I��V���зֽⷴӦ���ǣ���д������ţ���
�۲���11�е����������÷紵��ɹ����ɵģ�����ʵ�����н���������Ҫ����Ҫ������
��������ˮ��þ�������У���ȡ����Щ��ʩ�����ͳɱ���������Ⱦ�ģ���д������һ�㣺
��2���ش��������⣺
��ͼΪþԪ�ص�ij�����ӽṹʾ��ͼ����ͼ��ʾ������ţ�
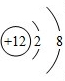
A������ B��ԭ�� C��������D�������ӣ�
��þԭ���ڻ�ѧ��Ӧ������ʧȥ���ӣ�þ��һ�֣�����á������á���
��3��Ϊ�ⶨԼ��þ30%��þ���Ͻ𣨲�������Ԫ�أ���þ������������
�ٵ�һС���������ʵ�鷽��������agþ���Ͻ��ĩ��������ͼ��ʾװ�õĶ��Ե��Ȱ��ϣ�ͨ��ʹ�������գ�
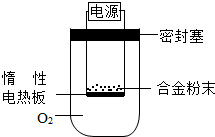
����һ��������þ��������������ʵ���л���ⶨ��һ��������
���۶������ÿ�������O2����ʵ�飬�Բⶨ�������С����ޡ���
�ڵڶ�С���������ʵ�鷽������ȡb gþ���Ͻ���������ϡ������ȫ��Ӧ��
������þ��������������ʵ���л���ⶨ��һ��������
��������1����Ҫ�ӻ�ѧ����ʽ����д�ͻ�����Ӧ���͵��жϻش𣺢���д��ѧ����ʽҪ��������У�������ȷд����Ӧ���������Ļ�ѧʽ���ٽ�����ƽ�����ע����Ӧ���������жϷ�Ӧ����Ҫ���ݷ�Ӧ���ص㣬�ֽⷴӦ���ص���һ�����ʱ�������ʣ��������������Ҫ�õ��������ܽ��ͳɱ���������Ⱦ�Ĵ�ʩҪ�Ӳ���ij�����ã���Լ��Դ���濼�ǣ�
��2��ԭ�ӵĽṹʾ��ͼ�������������Լ������������ͺ���������Ĺ�ϵ�������ӵ����ʺ����࣮
��3������֪þ���Ͻ�Ϊa�ˣ�þ���Ͻ�ȼ�պ���������þ�����������Ƴ���Ӧ����������Ϊb�ˣ����ǾͿ�����þ���Ͻ���þ������Ϊx������������Ϊ��a-x��g�������ɹ���������þ������Ϊy����������������Ϊ��b-y��g��Ȼ����ݻ�ѧ����ʽ�г�����ʽ���ⷽ������⼴�ɣ�
����֪þ���Ͻ�Ϊa�ˣ�þ���Ͻ������������������Ƴ���Ӧ����������������Ϊc�ˣ����ǾͿ�����þ���Ͻ���þ������Ϊm������������Ϊ��a-m��g����þ���ᷴӦ��������������Ϊn���������ᷴӦ��������������Ϊ��c-n��g��Ȼ����ݻ�ѧ����ʽ�г�����ʽ���ⷽ������⼴�ɣ�
��2��ԭ�ӵĽṹʾ��ͼ�������������Լ������������ͺ���������Ĺ�ϵ�������ӵ����ʺ����࣮
��3������֪þ���Ͻ�Ϊa�ˣ�þ���Ͻ�ȼ�պ���������þ�����������Ƴ���Ӧ����������Ϊb�ˣ����ǾͿ�����þ���Ͻ���þ������Ϊx������������Ϊ��a-x��g�������ɹ���������þ������Ϊy����������������Ϊ��b-y��g��Ȼ����ݻ�ѧ����ʽ�г�����ʽ���ⷽ������⼴�ɣ�
����֪þ���Ͻ�Ϊa�ˣ�þ���Ͻ������������������Ƴ���Ӧ����������������Ϊc�ˣ����ǾͿ�����þ���Ͻ���þ������Ϊm������������Ϊ��a-m��g����þ���ᷴӦ��������������Ϊn���������ᷴӦ��������������Ϊ��c-n��g��Ȼ����ݻ�ѧ����ʽ�г�����ʽ���ⷽ������⼴�ɣ�
����⣺��1����I���������ճ���ʯ�ҵĻ�ѧ����ʽΪ CaCO3
CaO+CO2����
��Mg��OH��2�����м��������кͷ�Ӧ�Ļ�ѧ����ʽΪ Mg��OH��2+2HCl=MgCl2+2H2O��
�ڷֽⷴӦ���ص���һ�����ʱ�������ʣ��������ձ��ǣ�������ˮ�Ȼ�þ�������ˮ�Ȼ�þ���ǷֽⷴӦ��
����������Ҫ�õ���Ҫ�������������������ƾ��ơ�����Ȧ������̨������ǯ�ȣ�
�ܽ��ͳɱ���������Ⱦ�Ĵ�ʩҪ�Ӳ���ij�����ã���Լ��Դ���濼�ǣ����������������������HCl
��2���ٴӽṹʾ��ͼ�п��������������Ⱥ���������࣬�����Ӵ�����ɣ��������ӣ���þԭ�ӵĽṹʾ��ͼ�У���������������ӣ���ʧȥ���ʻ�ѧ���ʻ��ã�
��3���ٲⶨþ���Ͻ���þ����������Ҫ����þ����������Ӧ���ɵ�������������ͬ�ⶨ���ڿ����вⶨ����Ϊþ�����뵪����������̼�����巴Ӧ����Ӱ������ȷ�ԣ�
��þ���Ͻ����������ᷴӦ�������ɵ�����������ͬ�ⶨ���ⶨþ������������
�𰸣���1����CaCO3
CaO+CO2�� Mg��OH��2+2HCl=MgCl2+2H2O
��I������V
�������� ������
�ܵ�������������������HCl ��2����C �ڻ���
��3������ȫ��Ӧ�����ɵĹ�������� ��
�ڳ�ַ�Ӧ�����ɵ������������������
| ||
��Mg��OH��2�����м��������кͷ�Ӧ�Ļ�ѧ����ʽΪ Mg��OH��2+2HCl=MgCl2+2H2O��
�ڷֽⷴӦ���ص���һ�����ʱ�������ʣ��������ձ��ǣ�������ˮ�Ȼ�þ�������ˮ�Ȼ�þ���ǷֽⷴӦ��
����������Ҫ�õ���Ҫ�������������������ƾ��ơ�����Ȧ������̨������ǯ�ȣ�
�ܽ��ͳɱ���������Ⱦ�Ĵ�ʩҪ�Ӳ���ij�����ã���Լ��Դ���濼�ǣ����������������������HCl
��2���ٴӽṹʾ��ͼ�п��������������Ⱥ���������࣬�����Ӵ�����ɣ��������ӣ���þԭ�ӵĽṹʾ��ͼ�У���������������ӣ���ʧȥ���ʻ�ѧ���ʻ��ã�
��3���ٲⶨþ���Ͻ���þ����������Ҫ����þ����������Ӧ���ɵ�������������ͬ�ⶨ���ڿ����вⶨ����Ϊþ�����뵪����������̼�����巴Ӧ����Ӱ������ȷ�ԣ�
��þ���Ͻ����������ᷴӦ�������ɵ�����������ͬ�ⶨ���ⶨþ������������
�𰸣���1����CaCO3
| ||
��I������V
�������� ������
�ܵ�������������������HCl ��2����C �ڻ���
��3������ȫ��Ӧ�����ɵĹ�������� ��
�ڳ�ַ�Ӧ�����ɵ������������������
�����������ۺ������ʵ��Ʊ�����ѧ����ʽ����д������ʵ�������ԭ�ӽṹʾ��ͼ��������гɷֵIJⶨ�ȶ�����㣬�ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ
 ��ͼ��ʾ������ţ�
��ͼ��ʾ������ţ�