��Ŀ����
��1����ͼ��ijͬѧ�Լ���Ƶĵ��ˮʵ��װ��ͼ1���ô�����ƿ�ӽ�ȥƿ�ף���ƿ��һ��Լ8cm��10cm��ƿ����һ������������������ A��B �������öƸ���������ֱ���ɣ�������¶ͷ�����ӵ��ߣ��Իش�
��ͼ1��֪B ��Ϊ
��2������ˮ��Դ����ֹˮ��Ⱦ��ÿ�������ȫ�������Σ��������������ڷ�ֹˮ��Դ��Ⱦ����
��ũҵ������Ҫ����ʹ��ũҩ�ͻ��� �ڹ�ҵ��ˮ��������ˮ�����������ŷ�
�۲��ú���ϴ�·� �ܽ���ˮ����
A���٢ڢ�B�� �ڢۢ�C���٢ڢ�D�� �٢ڢۢ�
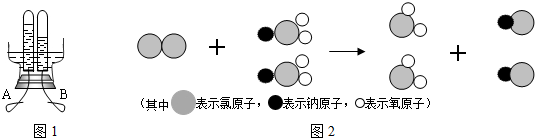
��3������ˮ�г���Ư�۶�����ˮ������������ClO2 ����Ϊ��һ������ˮ�������������������ҹ��ɹ����Ƴ���ȡClO2 ���·������䷴Ӧ���۹�����ʾ��ͼ2��
�Իش�ClO2 ����Ԫ�صĻ��ϼ�Ϊ

��ͼ1��֪B ��Ϊ
��
��
������A�������ӵ��Թ��еõ�������������
����
�����������ǵ�ľ��
�����ǵ�ľ��
�����飮��2������ˮ��Դ����ֹˮ��Ⱦ��ÿ�������ȫ�������Σ��������������ڷ�ֹˮ��Դ��Ⱦ����
C
C
������ţ�����ũҵ������Ҫ����ʹ��ũҩ�ͻ��� �ڹ�ҵ��ˮ��������ˮ�����������ŷ�
�۲��ú���ϴ�·� �ܽ���ˮ����
A���٢ڢ�B�� �ڢۢ�C���٢ڢ�D�� �٢ڢۢ�
��3������ˮ�г���Ư�۶�����ˮ������������ClO2 ����Ϊ��һ������ˮ�������������������ҹ��ɹ����Ƴ���ȡClO2 ���·������䷴Ӧ���۹�����ʾ��ͼ2��
�Իش�ClO2 ����Ԫ�صĻ��ϼ�Ϊ
+4
+4
�ۣ��������������Ӧ���۹���ʾ��ͼд����Ӧ�ķ��ű���ʽCl2+2NaClO2=2ClO2+2NaCl
Cl2+2NaClO2=2ClO2+2NaCl
����������1�����ݵ��ˮ����������۽��з��������ˮʱ���������ɵ����������õ����ǵ�ľ�����飻
��2�����ݷ�ֹˮ��Դ��Ⱦ�Ĵ�ʩ������
��3�����ݻ��ϼ۹����ڻ������и�Ԫ���������ϼ۵Ĵ�����Ϊ�������û�������ijԪ�صĻ��ϼۣ�����ͼʾ����д����Ӧ�Ļ�ѧ����ʽ��
��2�����ݷ�ֹˮ��Դ��Ⱦ�Ĵ�ʩ������
��3�����ݻ��ϼ۹����ڻ������и�Ԫ���������ϼ۵Ĵ�����Ϊ�������û�������ijԪ�صĻ��ϼۣ�����ͼʾ����д����Ӧ�Ļ�ѧ����ʽ��
����⣺��1���ɵ��ˮʵ��װ��ͼ1��֪��B�缫���ֻ�������϶࣬��������B�缫�ǵ�Դ�ĸ�������A�������ӵ��Թ��еõ���������٣�����������֧��ȼ�գ����ô����ǵ�ľ�������飻
��2����ҵ��ˮ��ũҵ��ˮ��������ˮ�������������ŷţ�������ʹ�û��ʺ�ũҩ�������ڷ�ֹˮ��Դ��Ⱦ�����ں���ϴ�·���ʹˮ��Ӫ��������ɳೱ��ˮ���������Բ��ú���ϴ�·������ڷ�ֹˮ��Ⱦ����ˮ������ˮ����Ⱦû��ֱ�ӹ�ϵ����C��ȷ��
��3���ڻ��������������ϼ۴�����Ϊ�㣬��ClO2 ����Ԫ�صĻ��ϼ�Ϊ-2�ۣ���Ԫ�صĻ��ϼ�Ϊ+4�ۣ��ɷ�Ӧ���۹���ͼ��֪����Ӧ�Ļ�ѧ����ʽΪ��Cl2+2NaClO2=2ClO2+2NaCl��
�ʴ�Ϊ����1�����������������ǵ�ľ������2��C����3��+4��Cl2+2NaClO2=2ClO2+2NaCl��
��2����ҵ��ˮ��ũҵ��ˮ��������ˮ�������������ŷţ�������ʹ�û��ʺ�ũҩ�������ڷ�ֹˮ��Դ��Ⱦ�����ں���ϴ�·���ʹˮ��Ӫ��������ɳೱ��ˮ���������Բ��ú���ϴ�·������ڷ�ֹˮ��Ⱦ����ˮ������ˮ����Ⱦû��ֱ�ӹ�ϵ����C��ȷ��
��3���ڻ��������������ϼ۴�����Ϊ�㣬��ClO2 ����Ԫ�صĻ��ϼ�Ϊ-2�ۣ���Ԫ�صĻ��ϼ�Ϊ+4�ۣ��ɷ�Ӧ���۹���ͼ��֪����Ӧ�Ļ�ѧ����ʽΪ��Cl2+2NaClO2=2ClO2+2NaCl��
�ʴ�Ϊ����1�����������������ǵ�ľ������2��C����3��+4��Cl2+2NaClO2=2ClO2+2NaCl��
������������Ҫ������ˮ����ɡ���ֹ��Ⱦ��������֪ʶ��ˮ��һ����Ҫ�����ʣ�Ӧ��ǿ�й�ˮ��֪ʶ��ѧϰ��

��ϰ��ϵ�д�
�����Ŀ
��ͼ��ijͬѧ�ڳ��������ʳ�ô����Ҫ�ɷ���Na2CO3����װ����Ϣ��������һ��ʳ�ô��ѧУʵ���ң�
��1��Ϊ�ⶨ������Na2CO3���ܽ�ȣ��ڱ��ΪA��B��C��D���ĸ��ձ��и����������µ�ˮ100g�����ֱ����ȡ��ʵ���ҵ�Na2CO3���壬����������ܽ⣬ʵ�����ݼ�¼�����
�����������ݣ��ձ����Ϊ______�е���Һ�DZ�����Һ�������£�Na2CO3���ܽ����
______��
��2��Ϊȷ��ʳ�ô���Ĵ��ȣ���ȡ�Դ���ʳ�ô���5.4g�����ձ��У��ٵμ��������պ���ȫ��Ӧ������ȥϡ����25g������������Ϊ28.2g����������ˮ���������Ӧ����ͨ�������жϸ�ʳ�ô�����̼���Ƶ����������Ƿ����װ����Ϣ���������������ȷ��0.1%��

��1��Ϊ�ⶨ������Na2CO3���ܽ�ȣ��ڱ��ΪA��B��C��D���ĸ��ձ��и����������µ�ˮ100g�����ֱ����ȡ��ʵ���ҵ�Na2CO3���壬����������ܽ⣬ʵ�����ݼ�¼�����
| �ձ���� | A | B | C | D |
| ˮ������/�� | 100 | 100 | 100 | 100 |
| ����Na2CO3������/�� | 30 | 35 | 40 | 50 |
| ��Һ������/�� | 130 | 135 | 140 | 140 |
______��
��2��Ϊȷ��ʳ�ô���Ĵ��ȣ���ȡ�Դ���ʳ�ô���5.4g�����ձ��У��ٵμ��������պ���ȫ��Ӧ������ȥϡ����25g������������Ϊ28.2g����������ˮ���������Ӧ����ͨ�������жϸ�ʳ�ô�����̼���Ƶ����������Ƿ����װ����Ϣ���������������ȷ��0.1%��

��ͼ��ijͬѧ�ڳ��������ʳ�ô����Ҫ�ɷ���Na2CO3����װ����Ϣ��������һ��ʳ�ô��ѧУʵ���ң�
��1��Ϊ�ⶨ������Na2CO3���ܽ�ȣ��ڱ��ΪA��B��C��D���ĸ��ձ��и����������µ�ˮ100g�����ֱ����ȡ��ʵ���ҵ�Na2CO3���壬����������ܽ⣬ʵ�����ݼ�¼�����
�����������ݣ��ձ����Ϊ______�е���Һ�DZ�����Һ�������£�Na2CO3���ܽ����
______��
��2��Ϊȷ��ʳ�ô���Ĵ��ȣ���ȡ�Դ���ʳ�ô���5.4g�����ձ��У��ٵμ��������պ���ȫ��Ӧ������ȥϡ����25g������������Ϊ28.2g����������ˮ���������Ӧ����ͨ�������жϸ�ʳ�ô�����̼���Ƶ����������Ƿ����װ����Ϣ���������������ȷ��0.1%��

��1��Ϊ�ⶨ������Na2CO3���ܽ�ȣ��ڱ��ΪA��B��C��D���ĸ��ձ��и����������µ�ˮ100g�����ֱ����ȡ��ʵ���ҵ�Na2CO3���壬����������ܽ⣬ʵ�����ݼ�¼�����
| �ձ���� | A | B | C | D |
| ˮ������/�� | 100 | 100 | 100 | 100 |
| ����Na2CO3������/�� | 30 | 35 | 40 | 50 |
| ��Һ������/�� | 130 | 135 | 140 | 140 |
______��
��2��Ϊȷ��ʳ�ô���Ĵ��ȣ���ȡ�Դ���ʳ�ô���5.4g�����ձ��У��ٵμ��������պ���ȫ��Ӧ������ȥϡ����25g������������Ϊ28.2g����������ˮ���������Ӧ����ͨ�������жϸ�ʳ�ô�����̼���Ƶ����������Ƿ����װ����Ϣ���������������ȷ��0.1%��

 ��2013?���ˣ���ͼ��ijͬѧ�ڳ��������ʳ�ô����Ҫ�ɷ���Na2CO3����װ����Ϣ��������һ��ʳ�ô��ѧУʵ���ң�
��2013?���ˣ���ͼ��ijͬѧ�ڳ��������ʳ�ô����Ҫ�ɷ���Na2CO3����װ����Ϣ��������һ��ʳ�ô��ѧУʵ���ң�