��Ŀ����
������һ����Ҫ�IJ��ϣ������������������벻����������ͼ��ʾ�˽������˳�����ͭ������������Ԫ�ر�������ģ���������õĴ������ޣ�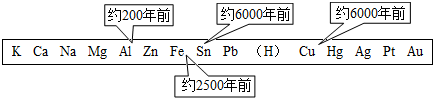
��1������ͼ�����ݺ��йصĻ�ѧ֪ʶ������Ϊ�������ģ���������õ��Ⱥ�˳���������Щ�����й�
�ٽ���ұ�������׳̶� �ڽ����ĵ����� �۽����Ļ�� �ܵؿ��н���Ԫ�صĺ���
��2��������������ʹ����������������������һ����Ҫ��־������д��һ����̼�ڸ��������°����ӳ������ﻹԭ�����Ļ�ѧ����ʽ

��3��ÿ�������ϸ����IJ����ܸߣ�����������ʴҲ����������˾����ʧ�����ڿ�������ʴ��ʵ����������
��4�����Ļ�Ա���ǿ�������������ȴ���ֳ����õĿ�ʴ�ԣ���ԭ��
��5������������֯�ѽ�������ΪʳƷ��ȾԴ֮һ��ָ��ÿ��ÿ�����İ�ȫ������Ӧ������4�������£�ij��ʳ����1000����ۡ�4������[��ѧʽΪKAl��SO4��2?12H2O]��С�մ�ʳ�εȸ��ϼ�����ˮ����ը������24����ƽ��ÿ��50�ˣ��ʣ�
������������Է�������Ϊ
�������м�Ԫ�ء���Ԫ�ء���Ԫ�ء���Ԫ�ء���Ԫ�ص�������Ϊ
����֪��������Ԫ�ص���������Ϊ5.7%����4����������Ԫ�ص�������
��������ը������������Ԫ�ز���ʧ��ij��һ���һ���������������������Ƿ���ȫ��������
�����پ�һ���ճ������лᵼ�������������;����
��������1������ͼ���������Ľ������ģ���������õ��Ⱥ�˳��������������˳��Ĺ�ϵ�������жϣ�
��2��������ұ��ԭ����д��һ����̼�ڸ��������°����ӳ������ﻹԭ�����Ļ�ѧ����ʽ��
��3�����ݶ�������֪ʶ�Ļعˣ�˵�������ԭ�������г��IJ�ͬ��λ��ֹ��ʴ�ķ�����
��4���������Ļ�Ա���ǿ���������ڿ�����ȴ���ֳ����õĿ�ʴ�Ե�ԭ��
��5������Է�������Ϊ����������ԭ�ӵ����ԭ�������ܺͣ�������Է���������
�����Ԫ��������Ϊ��Ԫ�ص����ԭ��������ԭ�Ӹ����˻��ıȣ��������Ԫ�������ȣ�
������һ������������Ԫ�ص�����=������������Ԫ����������������������Ԫ�ص���������Ϊ5.7%����4����������Ԫ�ص�������
�������������й����������ݣ�����ÿ����������Ԫ������������ȫ���Աȣ������жϣ�
�ݻ���������������ص���Ʒ������ʹ�������ǵ��������������;����
��2��������ұ��ԭ����д��һ����̼�ڸ��������°����ӳ������ﻹԭ�����Ļ�ѧ����ʽ��
��3�����ݶ�������֪ʶ�Ļعˣ�˵�������ԭ�������г��IJ�ͬ��λ��ֹ��ʴ�ķ�����
��4���������Ļ�Ա���ǿ���������ڿ�����ȴ���ֳ����õĿ�ʴ�Ե�ԭ��
��5������Է�������Ϊ����������ԭ�ӵ����ԭ�������ܺͣ�������Է���������
�����Ԫ��������Ϊ��Ԫ�ص����ԭ��������ԭ�Ӹ����˻��ıȣ��������Ԫ�������ȣ�
������һ������������Ԫ�ص�����=������������Ԫ����������������������Ԫ�ص���������Ϊ5.7%����4����������Ԫ�ص�������
�������������й����������ݣ�����ÿ����������Ԫ������������ȫ���Աȣ������жϣ�
�ݻ���������������ص���Ʒ������ʹ�������ǵ��������������;����
����⣺��1����ͼ�н������ģ���������õ��Ⱥ�˳�������ɵó������ģʹ�õ��Ⱥ���������ǿ���෴����˿��Ʋ���������ģ���������õ��Ⱥ�˳����ٽ���ұ�������׳̶Ⱥ͢۽����Ļ���йأ�
��2��һ����̼�ڸ�����������������Ӧ�������Ͷ�����̼����Ӧ�Ļ�ѧ����ʽ3CO+Fe2O3
2Fe+3CO2��
��3������������������е�ˮ�������������ӻ�ѧ�仯�Ľ�����ճ����������г����Ǽܲ�ȡ��Ϳ���ᡢ������ȡ��ơ�������ȡͿ�͵ķ������з�ֹ��ʴ��
��4�����Ļ�Ա���ǿ�������½������������������Ӧ�����ı����������ܵ���������Ĥ��������Ĥ������������������ĽӴ���
��5��������KAl��SO4��2?12H2O��Է�������=39+27+32��2+16��8+12��18=474
������KAl��SO4��2?12H2O�м�Ԫ�ء���Ԫ�ء���Ԫ�ء���Ԫ�ء���Ԫ�ص�������=39��27����32��2������16��20������1��24��=39��27��64��320��24
��4����������Ԫ�ص�����=4g��5.7%=0.228g
��һ�������к���������=8g��
=0.0095g=9.5mg��4mg��һ�������еĺ�����ԶԶ������4mg�İ�ȫ������
�����ƴ��ߡ�������ˮ����������θҩ�������ޡ�������װ�����ж��������������ܳ�Ϊ���������������;����
�ʴ�Ϊ��
��1���٢ۣ�
��2��3CO+Fe2O3
2Fe+3CO2��
��3��ˮ��������a��Ϳ���b��ƣ�cͿ�ͣ�
��4�����ı����������ܵ���������Ĥ��������Ĥ������������������ĽӴ���
��5����474����39��27��64��320��24����0.228g����һ�������еĺ�����ԶԶ������4mg�İ�ȫ�������������ƴ��ߣ���������ˮ����������θҩ�������ޡ�������װ�����е��κ�һ�֣���
��2��һ����̼�ڸ�����������������Ӧ�������Ͷ�����̼����Ӧ�Ļ�ѧ����ʽ3CO+Fe2O3
| ||
��3������������������е�ˮ�������������ӻ�ѧ�仯�Ľ�����ճ����������г����Ǽܲ�ȡ��Ϳ���ᡢ������ȡ��ơ�������ȡͿ�͵ķ������з�ֹ��ʴ��
��4�����Ļ�Ա���ǿ�������½������������������Ӧ�����ı����������ܵ���������Ĥ��������Ĥ������������������ĽӴ���
��5��������KAl��SO4��2?12H2O��Է�������=39+27+32��2+16��8+12��18=474
������KAl��SO4��2?12H2O�м�Ԫ�ء���Ԫ�ء���Ԫ�ء���Ԫ�ء���Ԫ�ص�������=39��27����32��2������16��20������1��24��=39��27��64��320��24
��4����������Ԫ�ص�����=4g��5.7%=0.228g
��һ�������к���������=8g��
| 1 |
| 24 |
�����ƴ��ߡ�������ˮ����������θҩ�������ޡ�������װ�����ж��������������ܳ�Ϊ���������������;����
�ʴ�Ϊ��
��1���٢ۣ�
��2��3CO+Fe2O3
| ||
��3��ˮ��������a��Ϳ���b��ƣ�cͿ�ͣ�
��4�����ı����������ܵ���������Ĥ��������Ĥ������������������ĽӴ���
��5����474����39��27��64��320��24����0.228g����һ�������еĺ�����ԶԶ������4mg�İ�ȫ�������������ƴ��ߣ���������ˮ����������θҩ�������ޡ�������װ�����е��κ�һ�֣���
�������ڸ��������Ļ�ѧʽ���м���ʱ������12H2Oǰ��ĵ�ֻ��һ�������ʾ��������Ҫ����������ѧ�ϳ˻��ļ�д���ţ�

��ϰ��ϵ�д�
�����Ŀ

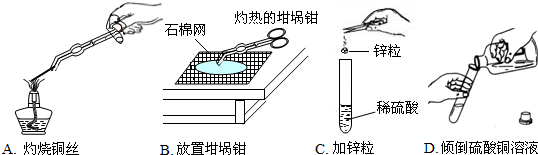
 ������һ����Ҫ�IJ��ϣ������������������벻��������
������һ����Ҫ�IJ��ϣ������������������벻��������