��Ŀ����
������NH3����һ����ɫ�д̼�����ζ�����壬��������ˮ������ˮ��Һ��Ϊ��ˮ���Լ��ԣ����ڻ�ѧ��ҵ����;�ܹ㷺�������ƻ��ʡ��ƴ���ȣ����������������ڻ���������
��1���������е�����Ԫ�ص�����֮��Ϊ________��
��2���������Ƽ���ؼ�һ���ķ�Ӧԭ���ɱ�ʾΪ��NH3+CO2+H2O+NaCl�TNaHCO3+A����������AҲ���������ʣ�A�е�Ԫ�ص���������Ϊ________��
��3����ϸ�������£��ð��������״��Ĺ�ҵ��ˮ��ʹ���Ϊ����N2��CO2���Ӷ�����Ի�������Ⱦ���йصķ�ӦΪ��6NH3+5CH3OH+12B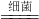 3N2��+5CO2��+19H2O��������Ӧ��B��Ӧ��������ʵĻ�ѧʽ��________��
3N2��+5CO2��+19H2O��������Ӧ��B��Ӧ��������ʵĻ�ѧʽ��________��
��4����400�����ң��д������ڵ������£��ð����ɽ��ж�����NO��ԭΪN2��H2O����д���÷�Ӧ�Ļ�ѧ����ʽ��________��
�⣺��1�����ݻ������и�Ԫ��������=��Ԫ�ص����ԭ��������ԭ�Ӹ���֮�ȣ��ɵð������е�����Ԫ�ص�����֮��Ϊ 14��3��
�ʴ�Ϊ 14��3��
��2�����������غ㶨�ɣ�ԭ�ӵ����ࡢ��Ŀ�ڻ�ѧ��Ӧǰ���֪��Ӧʽ��NH3+CO2+H2O+NaCl�TNaHCO3+A��������A��ѧʽΪ��NH4Cl��NH4Cl�е�Ԫ�ص���������Ϊ ��100%�T26.2%���ʴ�Ϊ��26.2%��
��100%�T26.2%���ʴ�Ϊ��26.2%��
��3�����������غ㶨�ɣ�ԭ�ӵ����ࡢ��Ŀ�ڻ�ѧ��Ӧǰ���֪��Ӧʽ6NH3+5CH3OH+12B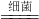 3N2��+5CO2��+19H2O��B�Ļ�ѧʽΪO2���ʴ�Ϊ��O2��
3N2��+5CO2��+19H2O��B�Ļ�ѧʽΪO2���ʴ�Ϊ��O2��
��4��������400�����ң��ڴ������ڵ������£��ð����ɽ��ж�����NO��ԭΪN2��H2O�����ݻ�ѧ����ʽ����д����ʽΪ��4NH3+6NO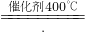 5N2+6H2O��
5N2+6H2O��
�ʴ�Ϊ��4NH3+6NO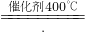 5N2+6H2O��
5N2+6H2O��
��������1�����ݻ������и�Ԫ��������=��Ԫ�ص����ԭ��������ԭ�Ӹ���֮�ȣ����н��
��2����3�����������غ㶨�ɣ�ԭ�ӵ����ࡢ��Ŀ�ڻ�ѧ��Ӧǰ�䣬���з������
��4�����ݻ�ѧ����ʽ����д�IJ��裺д��ע�ȣ�д����Ӧ����ʽ��
���������⿼��ѧ���Ի�������Ԫ�ص������ȣ������غ㶨�ɵ�Ӧ�ã�����ѧ����Ӧ��֪ʶ��������
�ʴ�Ϊ 14��3��
��2�����������غ㶨�ɣ�ԭ�ӵ����ࡢ��Ŀ�ڻ�ѧ��Ӧǰ���֪��Ӧʽ��NH3+CO2+H2O+NaCl�TNaHCO3+A��������A��ѧʽΪ��NH4Cl��NH4Cl�е�Ԫ�ص���������Ϊ
 ��100%�T26.2%���ʴ�Ϊ��26.2%��
��100%�T26.2%���ʴ�Ϊ��26.2%����3�����������غ㶨�ɣ�ԭ�ӵ����ࡢ��Ŀ�ڻ�ѧ��Ӧǰ���֪��Ӧʽ6NH3+5CH3OH+12B
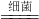 3N2��+5CO2��+19H2O��B�Ļ�ѧʽΪO2���ʴ�Ϊ��O2��
3N2��+5CO2��+19H2O��B�Ļ�ѧʽΪO2���ʴ�Ϊ��O2����4��������400�����ң��ڴ������ڵ������£��ð����ɽ��ж�����NO��ԭΪN2��H2O�����ݻ�ѧ����ʽ����д����ʽΪ��4NH3+6NO
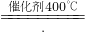 5N2+6H2O��
5N2+6H2O���ʴ�Ϊ��4NH3+6NO
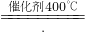 5N2+6H2O��
5N2+6H2O����������1�����ݻ������и�Ԫ��������=��Ԫ�ص����ԭ��������ԭ�Ӹ���֮�ȣ����н��
��2����3�����������غ㶨�ɣ�ԭ�ӵ����ࡢ��Ŀ�ڻ�ѧ��Ӧǰ�䣬���з������
��4�����ݻ�ѧ����ʽ����д�IJ��裺д��ע�ȣ�д����Ӧ����ʽ��
���������⿼��ѧ���Ի�������Ԫ�ص������ȣ������غ㶨�ɵ�Ӧ�ã�����ѧ����Ӧ��֪ʶ��������

��ϰ��ϵ�д�
�����Ŀ