��Ŀ����
��7�֣����������Ҫ�ɷ������������������ʲ����뷴Ӧ��Ҳ������ˮ������ҵ������������ȡ�������Ĺ�������ͼ��ʾ������ش��������⣺
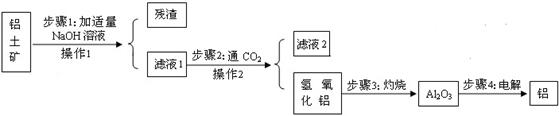
�Ų���1�з������·�Ӧ��Al2O3+2NaOH===2NaAlO2+X��X�Ļ�ѧʽ�� ��
�Ʋ���1�Ͳ���2�о��õ����������������� ��
�Dz���2�õ�����������������������θ����࣬����θ�ᷴӦ�Ļ�ѧ����ʽ�� ,�÷�Ӧ���� ��Ӧ������ϡ������ֽ⡱�����û����������ֽ⡱����
�Ȳ���3�з����ֽⷴӦ����������������������һ�ֳ��������������ɡ���д���÷�Ӧ�Ļ�ѧ����ʽ ��

�Ų���1�з������·�Ӧ��Al2O3+2NaOH===2NaAlO2+X��X�Ļ�ѧʽ�� ��
�Ʋ���1�Ͳ���2�о��õ����������������� ��
�Dz���2�õ�����������������������θ����࣬����θ�ᷴӦ�Ļ�ѧ����ʽ�� ,�÷�Ӧ���� ��Ӧ������ϡ������ֽ⡱�����û����������ֽ⡱����
�Ȳ���3�з����ֽⷴӦ����������������������һ�ֳ��������������ɡ���д���÷�Ӧ�Ļ�ѧ����ʽ ��
��H2O ������
��3HCl+Al(OH)3 AlCl3+3H2O ���ֽ�
��2Al(OH)3���� Al2O3+3H2O����Ӧ����д�ɡ�����Ҳ�ɣ�
��3HCl+Al(OH)3 AlCl3+3H2O ���ֽ�
��2Al(OH)3���� Al2O3+3H2O����Ӧ����д�ɡ�����Ҳ�ɣ�
���������
��1�����������غ㶨�ɻ�ѧ��Ӧǰ��ԭ�ӵ��������Ŀ�����֪����ӦǰAl��2��O��5��Na��2��H��2����Ӧ��Al��2��O��4��Na��2��H��0����X�Ļ�ѧʽ��H2O��
��2�������Һ�����ķ����ǹ��ˣ�����ʱ��Ҫ������������
��3���������������ᷴӦ���ɵ����Ȼ�����ˮ���������Ǹ��ֽⷴӦ��
��4�����ݻ�ѧ��Ӧǰ��Ԫ�ص�������֪����������ˮ��

��ϰ��ϵ�д�
 Сѧ��ʱ��ѵϵ�д�
Сѧ��ʱ��ѵϵ�д�
�����Ŀ