��Ŀ����
����Ŀ���Ͻ�����Ҫ�Ľ������ϣ�
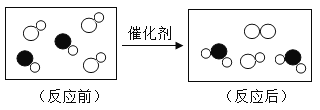
��1��������Ʒ��ʹ�õ���Ҫ�������ںϽ����_____������ĸ��ţ���ͬ����
A ���ƿ B ����ͧ C ����ֹ�
��2�������dz��õĺϽ���������_____�������������������� ����������
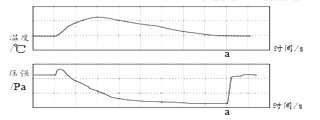
��3����ͭ��ͭп�Ͻ𣬽���ͭƬ�ͻ�ͭƬ����̻�����ͼ1��ʾ������ͭƬ���������ԵĻ��ۣ�˵��_____��
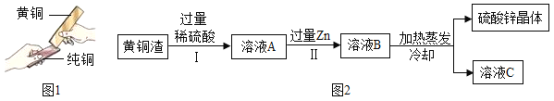
��4����ͭ����Լ�� Zn 7%��ZnO 31%��Cu 50%��CuO 5%������Ϊ���ʣ�������ͭ���ɵõ�����п������Ҫ������ͼ2�����ʲ�����ˮ�������뷴Ӧ����
��֪��ZnO+H2SO4�TZnSO4+H2O�� CuO+H2SO4�TCuSO4+H2O
�����з�Ӧ�Ļ�ѧ����ʽΪ_____��
������˵����ȷ����_____��
a���������еIJ�������������
b����ҺA��ZnSO4 ����������CuSO4
c����ҺA������С����ҺB
d����ҺC�����ʵ���������С����ҺB��
���𰸡�C ����� ��ͭ��Ӳ�ȱȴ�ͭ��Ӳ�ȴ� ![]() ��
��![]() abc
abc
��������
��1���ݺϽ�Ķ��弰�����ĺϽ������
��2���Ͻ����ڻ���
��3���Ͻ�Ҫ��������Ĵ�������Ӳ�ȴ�
��4���ݽ������ᡢ�ε���Һ��Ӧ�Ĺ�����д��ѧ����ʽ��
��1�����ƿ���ڹ����β��ϣ�����ͧ�����л��߷��Ӳ��ϣ�����ֹ����ںϽ�
��2�������dz��õĺϽ𣬺Ͻ����ڻ������������ڻ���
��3������ͭƬ�ͻ�ͭƬ����̻�����ͭƬ���������ԵĻ��ۣ�˵����ͭ��Ӳ�ȱȴ�ͭ��Ӳ�ȴ�
��4��������п�������ͭ��ǰ�棬��п�ȿ������ᷴӦ��Ҳ����������ͭ��Ӧ����Ӧ�Ļ�ѧ����ʽ�ֱ�Ϊ ![]() ��
�� ![]() ��
��
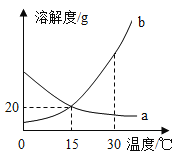
����ͼ��֪�����������˺��ͭ�������������dz�ȥп��������п��������������ͭ������ҺA������п��������������ͭ����ҺA����п��Ӧ�Ļ�ѧ����ʽ�ֱ�Ϊ![]() ��
��![]() �����ڷ�Ӧ
�����ڷ�Ӧ![]() ������֪��ÿ65��������п������Һ������Һ�г���2��������������ʹ����Һ�������ӣ����ڷ�Ӧ
������֪��ÿ65��������п������Һ������Һ�г���2��������������ʹ����Һ�������ӣ����ڷ�Ӧ![]() ��˵��ÿ65��������п������Һ������Һ������64��������ͭ��Ҳʹ����Һ�������ӣ�����ҺA������С����ҺB����ҺCΪ������Һ�����������ﵽͬ�¶��µ����ֵ����d����
��˵��ÿ65��������п������Һ������Һ������64��������ͭ��Ҳʹ����Һ�������ӣ�����ҺA������С����ҺB����ҺCΪ������Һ�����������ﵽͬ�¶��µ����ֵ����d����
�ʴ�Ϊ����1��C��2������3����ͭ��Ӳ�ȱȴ�ͭ��Ӳ�ȴ�4����![]() ��
��![]() ��abc��
��abc��

 ���������������Բ��������ϵ�д�
���������������Բ��������ϵ�д�����Ŀ��ʵ������һƿ���ܲ������Լ�����ͼ�������ȱ�ı�ǩ��ֻʣ����Na������10%����������֪������ɫҺ�壬�dz��л�ѧ���õ��Լ���С����С��ͬѧ�ܸ���Ȥ����������ɷֽ���̽����
��1�����������ǩ������жϣ���ƿ�Լ���������_____������ĸ���ţ���
A �� B �� C ��
��2����.ʵ��Ա˵����ƿ�Լ�������NaCl��NaOH��Na2CO3��NaHCO3������ʾ��Na2CO3�� NaHCO3��ˮ��Һ���Լ��ԡ�
��.�������ϣ����£�20����ʱ���������ʵ��ܽ�ȵ���������:
���� | NaCl | NaOH | Na2CO3 | NaHCO3 |
�ܽ��g | 36 | 109 | 215 | 9.6 |
���ó����ۣ�
С�������Լ�ƿ��ע��������������10%���ϱ��е��ܽ�ȵ������жϣ���ƿ�Լ���������_____����ͬѧ�ٴ�����۲��ų�����ͬѧ��Ϊ��������_____��
����Ʋ�ʵ�飩
�������ʵ��ȷ����ƿ�Լ������ƣ����������ʵ�鱨�档
��ʵ���ҽ��ṩ�˷�̪��ϡ���ᡢ����������Һ�Լ����õ�ʵ��������
ʵ�鲽�� | ʵ����������� |
_____ | _____ |
����Ŀ��3.0gij������ȫȼ�պ�����4.4gCO2��1.8gˮ����Ը���������ж���ȷ���ǣ�������
�� | A�� | ������ֻ��̼����Ԫ�� |
�� | B�� | ������һ������̼����Ԫ�أ����ܺ�����Ԫ�� |
�� | C�� | ��������̼���⡢��Ԫ����� |
�� | D�� | �����ʷ�����̼ԭ�Ӻ���ԭ�ӵĸ�����Ϊ1��1 |