��Ŀ����
 ����ͼ�ס�ͼ�һش��������⣺
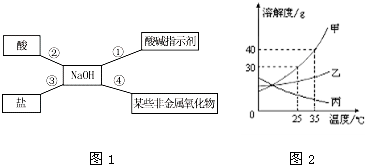
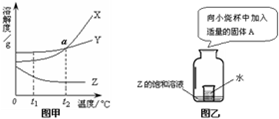
����ͼ�ס�ͼ�һش��������⣺��1��ͼ����X��Y��Z���ֹ������ʵ��ܽ�����ߣ�����a���ʾ�ĺ�����
��2������t2��ʱX���ʵIJ�������Һת��ɱ�����Һ���ɲ��õ�һ�ַ�����
��3����t2��ʱX��Y��Z�������ʱ�����Һ���¶Ƚ��͵�t1��ʱ��������Һ���������������ɴ�С��ϵ��
��4����ͼ����ʾ��20��ʱ��С�ձ��м��������Ĺ�������A����Z�ı�����Һ���в������������������Ĺ���A������
��2������X���ܽ�����¶ȱ仯���������������������������ԭ�����м��㣻
��3������X��Y��Z�������ʵ��ܽ�����¶ȱ仯�����������
��4������Z���ܽ�����¶ȱ仯�����������
��2����ͼʾ��֪��X���ʵ��ܽ�����¶������������ԣ�����t2��ʱX���ʵIJ�������Һת��ɱ�����Һ���ɲ��õ�һ�ַ����ǽ����¶ȣ�
������t1��ʱ100g 20% X��ҺŨ����40%����Һ��Ӧ����ˮ������Ϊx��
��������������ԭ���ã�
100g��20%=��100g-x����40%
��ã�x=50g
��3�����ܽ������ͼ���Կ�������t2��ʱ��X��Y��Z�������ʵ��ܽ�ȹ�ϵΪX=Y��Z�����ݱ�����Һ���������������ļ��㹫ʽ�������жϴ�ʱ������������ϵΪ��X=Y��Z�������µ�t1��ʱ��X��Y��Һ�о��о������������DZ�����Һ������t1��ʱ��X���ܽ�ȱ�Y�����Դ�ʱ������������Ϊ��X��Y��Z���ܽ�����¶Ƚ��Ͷ����߱�Ϊ��������Һ���������������������䣮������t1��ʱ��X��Y���ܽ�ȱ�t2��ʱZ���ܽ�ȴ�������Һ���������������ɴ�С��˳���ǣ�X��Y��Z��
��4�����ܽ������ͼ���Կ�����X���ʵ��ܽ�����¶������٣���ҪʹZ�ı�����Һ���в��������������������¶ȣ�����������ˮ��Ӧ�ų��������ȣ�ʹ��Һ���¶����ߣ����ԣ�����Ĺ������ʿ����������ƣ�
�ʴ�Ϊ����1����t2��ʱ��X��Y���ܽ����ͬ����2�������¶ȣ�50����3��X��Y��Z����4�������ƣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�(13��) ʵ���ҶԶ�����̼����ȡ�����ʵ�̽��
��1��ѡ��ҩƷ��С��������ҩƷ�������о���ʵ���¼���£�
| ��� | ҩƷ | ʵ������ |
| �� | ��״ʯ��ʯ��ϡ���� | ���������������� |
| �� | ��״ʯ��ʯ��ϡ���� | �����������ʻ�������ֹͣ |
| �� | ̼���Ʒ�ĩ��ϡ���� | �����������ʷdz��� |
����ȡ���ռ��ĽǶȷ�����һ��ѡ��ڢ���ҩƷ������ҩƷ������Ӧ�����ֱ���ʽΪ
����ѡ��ڢ���ҩƷ��ԭ���� ���Ҫ����������ɽ�����©����Ϊ____________(һ��ʵ������)��
��2��ѡ��װ�ã�ͨ������ȡ����װ�õķ�������ѡ���ù���������ȡ�����ķ���װ�ã���
��Ϊ��ѡ��������� �� ��
��3����ȡ���壮��ҩƷװ����ѡװ����ȡ���壬����____________________���ռ������������� ��
��4��������飮��ȼ�ŵ�ľ�����������У�ľ������Ϩ�������ȷ���������Ƕ�����̼��
���ļ��鷽���Ƿ���ȷ����˵�����ɣ� ��
��ȷ���������̼�ķ�����������Ӧ�����ֱ���ʽ��________________________________��
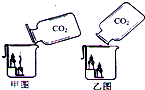
��5���������ʦ���ġ��㵹������̼������ʾʵ�飬�������ͼ�ش����⣺

�ס�����ͬѧ��ͬ�ռ�����ƿ������ͬ�Ķ�����̼���壬���ֱ�����̼�������������ձ��У�������ش�
�ټ�ͬѧ�ü�ͼ���㵹��ʽ���ձ����㵹�㹻�Ķ�����̼�����ǹ۲쵽�������������¶���Ϩ������������ǣ�Ϊʲô�����¶���Ϩ��
__________________________________________________��
����ͬѧ����ͼ���㵹��ʽ���ձ����㵹�㹻�Ķ�����̼�����ǹ۲쵽�Ľ������������û��Ϩ��������ϵѧ��֪ʶ ����������ͼ���㵹��ʽΪʲô������̼û�н����ձ���
__________________________________________________��
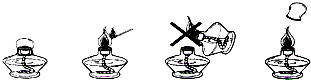
��6�������̲��С��ƾ��Ƶ�ʹ�÷����������۲�����ͼ�λش����⣺

��ֻ����ȼ�ŵĻ���ϸľ��ȥ��ȼ�ƾ��ƣ��þƾ�����ȼ��һֻ�ƾ��ƻ�����ʧ��ԭ����_______________________________________________��
��Ϊ��������ʧ������ƾ��ƺ���������ƾ��������Ӿƾ���Ӧ���õ�ñ������ʹ�ƾ���___________________________________�������Ӿƾ���
(13��) ʵ���ҶԶ�����̼����ȡ�����ʵ�̽��
��1��ѡ��ҩƷ��С��������ҩƷ�������о���ʵ���¼���£�
|
��� |
ҩƷ |
ʵ������ |
|
�� |
��״ʯ��ʯ��ϡ���� |
���������������� |
|
�� |
��״ʯ��ʯ��ϡ���� |
�����������ʻ�������ֹͣ |
|
�� |
̼���Ʒ�ĩ��ϡ���� |
�����������ʷdz��� |
����ȡ���ռ��ĽǶȷ�����һ��ѡ��ڢ���ҩƷ������ҩƷ������Ӧ�����ֱ���ʽΪ
����ѡ��ڢ���ҩƷ��ԭ���� ���Ҫ����������ɽ�����©����Ϊ____________(һ��ʵ������)��
��2��ѡ��װ�ã�ͨ������ȡ����װ�õķ�������ѡ���ù���������ȡ�����ķ���װ�ã���
��Ϊ��ѡ��������� �� ��
��3����ȡ���壮��ҩƷװ����ѡװ����ȡ���壬����____________________���ռ������������� ��
��4��������飮��ȼ�ŵ�ľ�����������У�ľ������Ϩ�������ȷ���������Ƕ�����̼��
���ļ��鷽���Ƿ���ȷ����˵�����ɣ� ��
��ȷ���������̼�ķ�����������Ӧ�����ֱ���ʽ��________________________________��
��5���������ʦ���ġ��㵹������̼������ʾʵ�飬�������ͼ�ش����⣺

�ס�����ͬѧ��ͬ�ռ�����ƿ������ͬ�Ķ�����̼���壬���ֱ�����̼�������������ձ��У�������ش�
�ټ�ͬѧ�ü�ͼ���㵹��ʽ���ձ����㵹�㹻�Ķ�����̼�����ǹ۲쵽�������������¶���Ϩ������������ǣ�Ϊʲô�����¶���Ϩ��
__________________________________________________��
����ͬѧ����ͼ���㵹��ʽ���ձ����㵹�㹻�Ķ�����̼�����ǹ۲쵽�Ľ������������û��Ϩ��������ϵѧ��֪ʶ ����������ͼ���㵹��ʽΪʲô������̼û�н����ձ���
__________________________________________________��
��6�������̲��С��ƾ��Ƶ�ʹ�÷����������۲�����ͼ�λش����⣺

��ֻ����ȼ�ŵĻ���ϸľ��ȥ��ȼ�ƾ��ƣ��þƾ�����ȼ��һֻ�ƾ��ƻ�����ʧ��ԭ����_______________________________________________��
��Ϊ��������ʧ������ƾ��ƺ���������ƾ��������Ӿƾ���Ӧ���õ�ñ������ʹ�ƾ���___________________________________�������Ӿƾ���