��Ŀ����
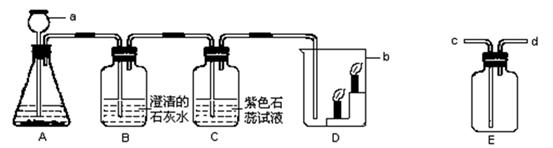
��6�֣���ͼ��ʾΪʵ�����г��������Ʊ�������ռ�������ʵ��IJ�����������װ
ʵ��װ��ʱ�����ظ�ѡ�����������Ը�����ĿҪ�ش��������⣺
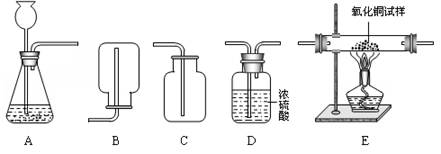
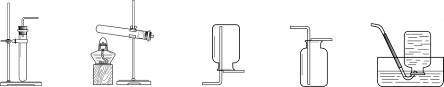
��1������H2O2��ҺΪԭ�ϣ���MnO2Ϊ��������ʵ�������Ʊ����ռ������������
����ѡ����������˳��Ϊ ����д���������ĸ����
����������ʱ������A�з����Ļ�ѧ��Ӧ����ʽ�� ��
��ʵ����Ҳ��������ˮ���ռ���������ԭ����__ ______��
��2������п��ϡ���ᷴӦ��ȡ�������������ⶨij��������������Ʒ�Ĵ��ȣ�����Ϊ����������������ѡ����������˳��Ϊ��A��D1��E��D2��D3������֪��Fe2O3+3H2��2Fe+3H2O��D1��D2��D3Ϊ3��ʢ��Ũ�����ϴ��ƿ��
������A�з����Ļ�ѧ��Ӧ����ʽ�� ��
������D1�������� ��
�۳�ַ�Ӧ��ϴ��ƿD2������������3.6g����Eװ���ڹ������������� g��
ʵ��װ��ʱ�����ظ�ѡ�����������Ը�����ĿҪ�ش��������⣺

��1������H2O2��ҺΪԭ�ϣ���MnO2Ϊ��������ʵ�������Ʊ����ռ������������
����ѡ����������˳��Ϊ ����д���������ĸ����
����������ʱ������A�з����Ļ�ѧ��Ӧ����ʽ�� ��
��ʵ����Ҳ��������ˮ���ռ���������ԭ����__ ______��
��2������п��ϡ���ᷴӦ��ȡ�������������ⶨij��������������Ʒ�Ĵ��ȣ�����Ϊ����������������ѡ����������˳��Ϊ��A��D1��E��D2��D3������֪��Fe2O3+3H2��2Fe+3H2O��D1��D2��D3Ϊ3��ʢ��Ũ�����ϴ��ƿ��
������A�з����Ļ�ѧ��Ӧ����ʽ�� ��
������D1�������� ��
�۳�ַ�Ӧ��ϴ��ƿD2������������3.6g����Eװ���ڹ������������� g��
����6�֣�ÿ��1�֣���1����A��D��C ��2H2O2 MnO22H2O+ O2����������������ˮ��2����Zn+H2SO4=ZnSO4+H2���ڸ��������������������е�ˮ�֣���3.2
�����ܶȱȿ���������Ҫѡ��Cװ�����ռ�������Ҫ���ռ������������������������Ҫ�õ�Dװ��ʹ��Ũ����������һ�£��ʣ�1������A��D��C
��ȡ�����Ļ�ѧ����ʽ��Ҫ��ǵģ�2H2O2 MnO22H2O+ O2��
��Ϊ������������ˮ�����Կ�������ˮ�����ռ��������ʣ�1������������������ˮ
��ȡ�����Ļ�ѧ����ʽ��Ҫ��ǵģ�Zn+H2SO4=ZnSO4+H2��
D1�����������������е�ˮ�֣�����IJⶨ��ͨ��ˮ�����ⶨ�ģ�����D1�в���ȥˮ��Ӱ��ⶨ�����
D2������������3.6g���Ƿ�Ӧ���ɵ�ˮ��������Eװ���ڹ�����ٵ�������������������Ԫ�ص�������Ҳ��������ˮ����Ԫ�ص������������У�3.6*16/18=3.2�ˣ�
��ȡ�����Ļ�ѧ����ʽ��Ҫ��ǵģ�2H2O2 MnO22H2O+ O2��
��Ϊ������������ˮ�����Կ�������ˮ�����ռ��������ʣ�1������������������ˮ
��ȡ�����Ļ�ѧ����ʽ��Ҫ��ǵģ�Zn+H2SO4=ZnSO4+H2��
D1�����������������е�ˮ�֣�����IJⶨ��ͨ��ˮ�����ⶨ�ģ�����D1�в���ȥˮ��Ӱ��ⶨ�����
D2������������3.6g���Ƿ�Ӧ���ɵ�ˮ��������Eװ���ڹ�����ٵ�������������������Ԫ�ص�������Ҳ��������ˮ����Ԫ�ص������������У�3.6*16/18=3.2�ˣ�

��ϰ��ϵ�д�
�����Ŀ
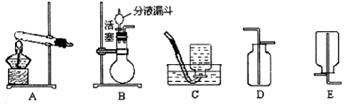
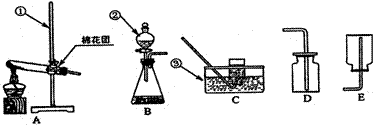
 ��ȡ������Ӧ�����ֱ���ʽ���Ȼ��+��ʯ�� ���� �Ȼ���+����+ˮ������ȡ���ռ�������Ӧ�ô���ͼ��ѡ��ķ���װ����________(�����)���ռ�װ����_________(�����)��
��ȡ������Ӧ�����ֱ���ʽ���Ȼ��+��ʯ�� ���� �Ȼ���+����+ˮ������ȡ���ռ�������Ӧ�ô���ͼ��ѡ��ķ���װ����________(�����)���ռ�װ����_________(�����)��