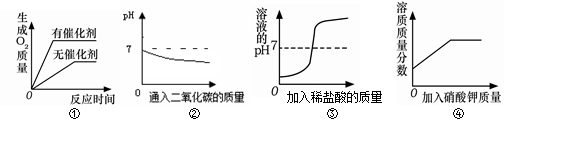
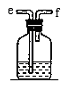��Ŀ����
��7�֣�ʵ���ҳ���һ�����������Ĺ���������ɫ��Һ������ΪH2O2�����ڶ�����������������������ȡ������ijͬѧʵ��ǰ��ù���������Һ42.5g������1 g MnO2����ȫ��Ӧ���÷�Ӧ������ʣ��������Ϊ41.9g�� ��ش�
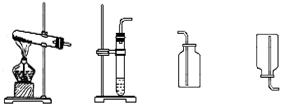
��1��ʵ�����÷ֽ�˫��ˮ�ķ�����ȡ����ʱ��ѡ��ķ���װ��ӦΪ_________��
��2���ռ�װ��Ӧѡ��_________��ԭ����____________________________________��
��3����Ӧ��ų������������Ƕ��٣����ù���������Һ�����ʵ����������Ƕ��٣�
[����������һλС����������̰�����д�ڴ��ֽ��]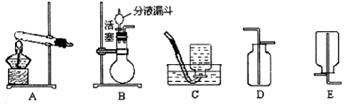
��1��ʵ�����÷ֽ�˫��ˮ�ķ�����ȡ����ʱ��ѡ��ķ���װ��ӦΪ_________��
��2���ռ�װ��Ӧѡ��_________��ԭ����____________________________________��
��3����Ӧ��ų������������Ƕ��٣����ù���������Һ�����ʵ����������Ƕ��٣�
[����������һλС����������̰�����д�ڴ��ֽ��]

��1�� B ����2�� C��D �� ������������ˮ�ұȿ������ܶȴ� ��
��3��[����������һλС����������̰�����д�ڴ��ֽ��]
�⣺��m����=" 42.5g" +1g ��41.9g="1.6g" ............................... 1��
�������������Һ�����ʵ�����ΪX����
2H2O2 ="=====" 2H2O + O2�� .............................. 1��
68 32
X 1.6g
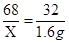 ��X =3.4g..................... 1��
��X =3.4g..................... 1��
�����������Һ�����ʵ���������="3.4g/42.5g" ��100%=8% ...... 1��
�𣺹���������Һ�����ʵ���������Ϊ8%
��3��[����������һλС����������̰�����д�ڴ��ֽ��]
�⣺��m����=" 42.5g" +1g ��41.9g="1.6g" ............................... 1��
�������������Һ�����ʵ�����ΪX����
2H2O2 ="=====" 2H2O + O2�� .............................. 1��
68 32
X 1.6g
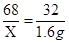 ��X =3.4g..................... 1��
��X =3.4g..................... 1�������������Һ�����ʵ���������="3.4g/42.5g" ��100%=8% ...... 1��
�𣺹���������Һ�����ʵ���������Ϊ8%
����˫��ˮ�ֽ�ķ�Ӧԭ�����������ܶȺ��ܽ���ȷ���������ռ�װ�ã������������غ㶨���ж����������������ݷ���ʽ�����ʵ����������������������Һ�����ʵ�����������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ