��Ŀ����
��8�֣�������������ʹ����������������������һ����Ҫ�ı�־����1��ÿ�������ʴ������ɾ����ʧ������Ʒ��ʴ����Ҫԭ����
��
��2�� У������ȤС���ͬѧ��ȥΫ��ij������������Ʒ����ʵ�飺���ķݲ�ͬ������������Ʒ���ٶ�����ֻ���������͵���̼���ֱ�ӵ�100g����������ͬ��ϡ�����У���ַ�Ӧ��õ�ʵ���������±�����֪���ڱ�״���£�22.4LH2������Ϊ2g��
| ʵ����� | 1 | 2 | 3 | 4 |
| ������Ʒ������ / g | 2.88 | 5.76 | 9.24 | 10.28 |
| ����H2���������״���£�/L | 1.12 | 2.24 | 3.36 | m |
������������m��ֵΪ ��
�ڸ��ݱ������ݼ���ϡ������H2SO4������������
(1)��������е�������ˮ����������Ӧ
��2����3.36
�ڽ⣺��ʵ��3���м���
����H2��������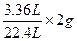 ��0.3g
��0.3g
������H2SO4������Ϊx
Fe+H2SO4==FeSO4+H2��
98 2
X 0.3g
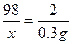
X=14.7g
ϡ������H2SO4����������Ϊ��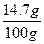 ��100%=14.7%
��100%=14.7%
�𣺣��ԣ�����:
��
��2����3.36
�ڽ⣺��ʵ��3���м���
����H2��������
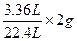 ��0.3g
��0.3g������H2SO4������Ϊx
Fe+H2SO4==FeSO4+H2��
98 2
X 0.3g
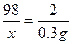
X=14.7g
ϡ������H2SO4����������Ϊ��
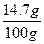 ��100%=14.7%
��100%=14.7%�𣺣��ԣ�����:
��

��ϰ��ϵ�д�
 ѧ���쳵�����ּ��ں�����ҵϵ�д�
ѧ���쳵�����ּ��ں�����ҵϵ�д�
�����Ŀ



