��Ŀ����
����С���ͬѧ�ڲⶨ��NaCl��Na2CO3�γɵĹ����������ʱ������������ʵ�飺ȡ40 g�������������Һ��ƽ����Ϊ�ķݣ�Ȼ��ֱ����һ������������CaCl2��Һ��ʵ�����ݼ��±���
| ʵ��һ | ʵ��� | ʵ���� | ʵ���� | |
| ԭ������������ | ����g | ����g | ����g | ����g |
| ����CaCl2��Һ���� | ����g | ����g | ����g | ����g |
| ���ɵij��������� | ��g | �� | ��g | ��g |
������������ݻش���[��4��Ҫ��д������� ]
��1�����ɵij����ǣ���д��ѧʽ�� �� ��2��10gԭ����������ɵ���Һ������CaCl2��Һ��Ӧ��������ɳ�������Ϊ g��
��3����= g��
��4��ԭ����������NaCl�����������Ƕ��٣�
(1)CaCO3 ��1�֣� (2)5 ��1�֣� (3) 4 ��1�֣�
��4��CaCl2 + Na2CO3 = CaCO3�� + 2NaCl
106 100
x 5g �������������������������������������� ��1�֣�
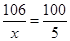 �������������������������������������� ��1�֣�
�������������������������������������� ��1�֣�
x = 5.3g ���������������������� ��1�֣�
����������NaCl������������: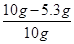 �� 100% = 47%
�� 100% = 47%
�������������� ��2�� ����ʽ�ͽ����1�֣�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| ʵ��һ | ʵ��� | ʵ���� | ʵ���� | |
| ԭ������������ | 10g | 10g | 10g | 10g |
| ����CaCl2��Һ���� | 10g | 20g | 30g | 40g |
| ���ɵij��������� | 2g | m | 5g | 5g |
��1��m=
��2��ԭ����������Na2CO3�����������Ƕ��٣�
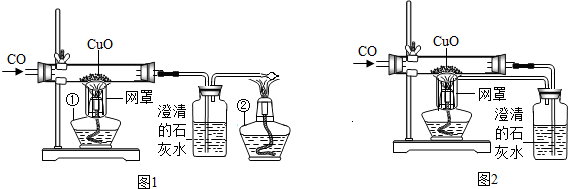
 ijУ����С���ͬѧ����ʦ�İ����£�̽���ó�������Ҫ�ɷ�Fe2O3����������Ҫ��Ӧԭ����������Ƶ�ʵ��װ�ã���ͼ��
ijУ����С���ͬѧ����ʦ�İ����£�̽���ó�������Ҫ�ɷ�Fe2O3����������Ҫ��Ӧԭ����������Ƶ�ʵ��װ�ã���ͼ��