��Ŀ����
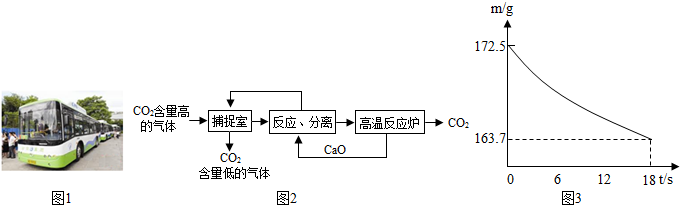
��̼����������ָͨ��һ���ķ���������ҵ�����в�����CO2����������д�������ã�������NaOH��Һ��������CO2��������ͼ��ʾ����������������δ
������������йظ÷�������������ȷ���ǣ�������

������������йظ÷�������������ȷ���ǣ�������

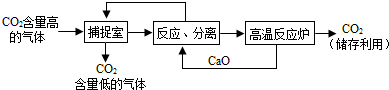
���������ռ�����������̼�����ڲ��ң�����̼���������������÷����ڷ����ң�ͬʱ�������ռ̼��Ƹ��·ֽ����������ƺͶ�����̼�����ڸ���¯������������CaO��NaOHѭ��������Ӧ���롱�����У�Ӧ�����ȹ��ˣ���Һ����ҪŨ���ᾧ��ֱ��ѭ��ʹ�ã�
����⣺A��̼��Ƶķֽ��ڸ��������½��У�������������A����
B���������������̿�֪����û������������룬Ҳû�ж�����̼�����������������Ը÷����õ��Ķ�����̼�Ǵ����ģ���B��ȷ��
C��������������������Ӧ���ٶ�����̼���������Ʒ�Ӧ����̼��Ƶĸ��·ֽ⣬ѭ�����õ�Ӧ����CaO��NaOH �������ʣ���C����
D������Ӧ���롱�����з������ʵIJ���Ӧ���ǹ��ˣ�Ŀ����ͨ�����˵õ�̼��Ƴ�������C����
��ѡB��
B���������������̿�֪����û������������룬Ҳû�ж�����̼�����������������Ը÷����õ��Ķ�����̼�Ǵ����ģ���B��ȷ��
C��������������������Ӧ���ٶ�����̼���������Ʒ�Ӧ����̼��Ƶĸ��·ֽ⣬ѭ�����õ�Ӧ����CaO��NaOH �������ʣ���C����
D������Ӧ���롱�����з������ʵIJ���Ӧ���ǹ��ˣ�Ŀ����ͨ�����˵õ�̼��Ƴ�������C����
��ѡB��
���������⿼����ѧ�������ʵ����ʺ�����ͼ�ķ�����ע�ض�ѧ������������Ŀ��飬����������ѧ����̽��������

��ϰ��ϵ�д�
�����Ŀ